Physicochemical properties and cytochromes P-450 kinetics of the trifluoroacetamido derivative of phenacetin
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
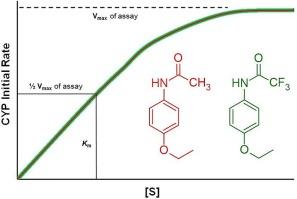
Substituting hydrogen atoms with fluorine alters physicochemical properties often resulting in improved drug action relative to the parent molecule. The high electronegativity of fluorine changes the electron density distribution of the molecule; however, the substitution does not significantly change the size of the molecule because hydrogen and fluorine have similar atomic radii. A trifluoroacetamido derivative (TFA-phenacetin) of phenacetin, an analgesic antipyretic drug, was synthesized to compare its lipophilicity to the parent molecule by determining octanol–water partition coefficients. TFA-phenacetin is over seven times more lipophilic than phenacetin, which suggests that TFA-phenacetin would have better bioavailability relative to phenacetin. The kinetics of cytochromes P-450 (CYP) catalyzed oxidation of phenacetin and TFA-phenacetin were compared using Sprague Dawley (SD) rat liver microsomes. Phenacetin and TFA-phenacetin have the same apparent binding affinity for the SD rat liver microsome CYP proteome and undergo CYP catalyzed oxidation at the same rate in the presence of SD rat liver microsomes.
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


