2-Amino-6-methylbenzothiazole as corrosion inhibitor for low carbon steel in acidic solution: Experimental and theoretical studies
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
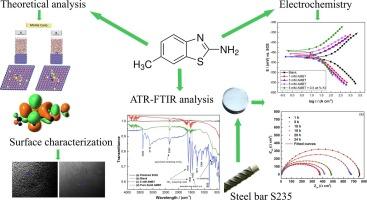
The corrosion susceptibility of S235 steel samples in 1 M HCl solution, with and without addition of 2-amino-6-methylbenzothiazole (AMBT) was evaluated using weight loss (WL), chronopotentiometry, electrochemical impedance spectroscopy (EIS), and potentiodynamic polarization (PD) techniques. The corrosion inhibition efficiency of AMBT was investigated at 298 to 318 K, with and without addition of 0.5 wt.% KI. The highest corrosion inhibition efficiency (i.e. 83.30%) was achieved upon addition of 5 mM AMBT. PD curve measurements after 24 h immersion revealed that AMBT behaves as a mixed-type inhibitor, predominantly affecting the cathodic corrosion reaction. Thermodynamic calculations showed that AMBT adheres to the Langmuir adsorption isotherm. Attenuated total reflectance Fourier transform infrared (ATR-FTIR) was used to confirm the adsorption of AMBT, while its influence on the morphology of the S235 steel samples was also investigated by scanning electron microscopy (SEM). Density Functional Theory (DFT) calculations, along with Monte Carlo (MC) and Molecular Dynamics (MD) simulations, were employed to investigate AMBT’s corrosion inhibition behaviour at the molecular level. The simulations confirmed that AMBT strongly adsorbs on the Fe(110) surface through a combination of physisorption and chemisorption mechanisms. This study offers detailed insights into AMBT’s effectiveness as a corrosion inhibitor.
2-氨基-6-甲基苯并噻唑作为低碳钢在酸性溶液中的缓蚀剂:实验与理论研究
采用失重法(WL)、时电位法、电化学阻抗谱法(EIS)和动电位极化法(PD)评价了S235钢样品在1 M HCl溶液中添加和不添加2-氨基-6-甲基苯并噻唑(AMBT)的腐蚀敏感性。在298 ~ 318 K的温度下,研究了添加和不添加0.5 wt.% KI时AMBT的缓蚀效率。添加5 mM AMBT时,缓蚀效率最高(83.30%)。浸泡24 h后的PD曲线测量显示,AMBT表现为混合型缓蚀剂,主要影响阴极腐蚀反应。热力学计算表明,AMBT符合Langmuir吸附等温线。采用衰减全反射傅里叶变换红外光谱(ATR-FTIR)证实了AMBT的吸附作用,并通过扫描电镜(SEM)研究了AMBT对S235钢试样形貌的影响。采用密度泛函理论(DFT)计算,以及蒙特卡罗(MC)和分子动力学(MD)模拟,研究了AMBT在分子水平上的缓蚀行为。模拟结果证实,AMBT通过物理吸附和化学吸附的结合机制在Fe(110)表面进行了强吸附。这项研究为AMBT作为缓蚀剂的有效性提供了详细的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


