Preparation and characterization of the ground mixture of rebamipide commercial tablets and Hydroxypropyl Cellulose-SSL by ball-milling: Application to the dispersoid of mouthwash suspension
IF 4.4
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-11-27
DOI:10.1016/j.ejpb.2024.114584
引用次数: 0
Abstract
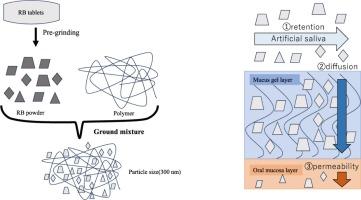
In the present study, to prepare dispersoids with high dispersion stability that can be used as mouthwash, ground mixtures of commercial rebamipide (RB) tablets and hydroxypropyl cellulose (HPC-SSL) samples were prepared by dry milling. The physicochemical properties of the ground mixture of HPC-SSL and the powder obtained from the preliminary ground RB tablets were then compared. The dispersoids’ physicochemical properties, dispersion stability, retention, and diffusiveness to the mucosal surfaces were evaluated in vitro. By co-grinding with HPC-SSL, RB transformed into fine particles around 303.6 – 361.5 nm that tended to prevent particle agglomeration in solution over time. The sample microparticles formed with HPC-SSL also avoided agglomeration on the mucosal surface for 24 h, improving oral retention. Furthermore, the ground mixture of HPC-SSL and the powder obtained from the preliminary ground RB tablets dispersed and permeated the mucus gel layer on the mucosal surface (24.2 %) but not the mucosal cells, indicating that RB remained on the mucosal surface. These results suggest that ground mixtures of commercial rebamipide (RB) tablets and hydroxypropyl cellulose (HPC-SSL) samples can be applied as a powdered suspension preparation because they showed high dispersion stability and oral mucosal retention.
球磨法制备利巴米胺市售片与羟丙基纤维素- ssl混料及其在漱口水混悬液中的应用
为了制备具有高分散稳定性的漱口水分散体,采用干磨法制备了利巴米胺(RB)片与羟丙基纤维素(HPC-SSL)样品的混合物。然后比较了HPC-SSL研磨混合物与RB片初磨粉末的理化性质。体外评价了分散体的理化性质、分散稳定性、保留率和对粘膜表面的弥漫性。通过与HPC-SSL共磨,RB转化为303.6 ~ 361.5 nm左右的细颗粒,随着时间的推移,颗粒在溶液中趋于不团聚。HPC-SSL形成的样品微颗粒在24 h内避免了在粘膜表面的团聚,提高了口腔潴留。此外,HPC-SSL的研磨混合物和初磨RB片的粉末分散并渗透到粘膜表面的粘液凝胶层(24.2%),但未渗透到粘膜细胞,表明RB仍留在粘膜表面。这些结果表明,利巴米胺(RB)片和羟丙基纤维素(HPC-SSL)样品的研磨混合物可以作为粉末悬浮液制备,因为它们具有高分散稳定性和口腔粘膜保留性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


