Reaching medium entropy oxides with rocksalt structure based on cluster-plus-glue-atom model: A case study of Co–Ni–Cu–Zn–O system with tunable optoelectrical properties
IF 3.2
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
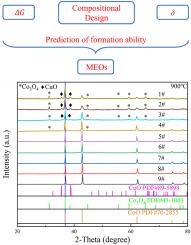
Mg–Co–Ni–Cu–Zn–O is the firstly reported high entropy oxide (HEO) which has great promise in optoelectrical applications. However, the multicomponent and decreased configurational entropy by extracting one element from (Mg, Co, Ni, Cu, Zn) makes it difficult to synthesize single-phase medium entropy oxides (MEOs) especially when the cations are non-equimolar. In this paper, we synthesized different compositional-type of non-equimolar Co–Ni–Cu–Zn–O MEOs possessing single-phase rocksalt structure guided by the cluster-plus-glue-atom model. Interestingly, Co21Ni43Cu16Zn16O96 and Co17Ni47Cu16Zn16O96 have the lowest single-phase formation temperature (850 °C) when the total cation content is kept unchanged. Further increase or decrease of Co/Ni content (none, little, more) will increase the difficulty of single-phase formation ability and temperature (900–1200 °C). It is suggested the single-phase formation ability of Co–Ni–Cu–Zn–O MEOs is closely related to the Gibbs free energy (ΔG) and average cation-size difference (δ). The linear decrease of Co content and increase of Ni content can monotonously enlarge the energy band gap (Eg) from 1.52 eV to 1.93 eV and increase the electrical impedance with five orders of magnitude (∼103 Ω–∼108 Ω), which may be valuable in the fields of optoelectrical devices.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Solid State Chemistry
化学-无机化学与核化学
CiteScore
6.00
自引率
9.10%
发文量
848
审稿时长
25 days
期刊介绍:
Covering major developments in the field of solid state chemistry and related areas such as ceramics and amorphous materials, the Journal of Solid State Chemistry features studies of chemical, structural, thermodynamic, electronic, magnetic, and optical properties and processes in solids.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


