Magnetic MXene chitosan-lignosulfonate composite (Fe3O4@ MCLS) for the reductive removal of Cr(VI) and other heavy metals from water
IF 5.4
Q2 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
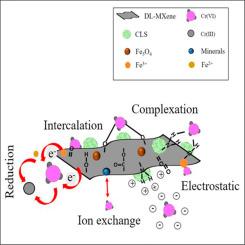
In this study, magnetic MXene (Ti3C2Tx) chitosan-lignosulfonate composite (Fe3O4@MCLS), was synthesized based on the facile integration of Fe3O4, chitosan-lignosulfonate (CLS) nanospheres and delaminated (DL) Ti3C2Tx. This composite was designed to integrate the biocompatibility of CLS and selective adsorption of MXene with the benefit of magnetic separation. Characterization confirmed the successful stabilization of the magnetic chitosan-lignosulfonate on the MXene surface, resulting in multiple surface functionalization groups and a high specific surface area. Fe3O4@MCLS was initially tested for the removal of Cr(VI) in a batch-system, achieving 90% efficiency and a capacity of 42.5 mg/g at neutral pH. Adsorption kinetics followed the Pseudo-second-order model, and equilibrium data fit the Langmuir isotherm, indicating a monolayer adsorption mechanism. The composite demonstrated high selectivity towards Cr(VI) ions and improved magnetic recovery from the media. The results suggested prevalent adsorption mechanisms included electrostatic interactions, complexation, surface intercalation, and reduction of toxic Cr(VI) to Cr(III) on the composite adsorbent. Further validation of the composite's performance was carried out through a competitive adsorption study in a multi-metal system. The results showed that the composite effectively removed heavy metals, exhibiting varying affinities for different metal ions, following the trend: Cr(VI) > Ni(II) > Cu(II) ≈ Co(II) under neutral pH conditions. Overall, the present study demonstrates the facile preparation of a new composite material, which exhibits sustainable characteristics due to the incorporation of chitosan-lignosulfonate and iron oxide. This eco-friendly and recyclable composite shows significant potential for application in water treatment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of hazardous materials advances
Environmental Engineering
CiteScore
4.80
自引率
0.00%
发文量
0
审稿时长
50 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


