Effects of Ce/Ca/Mg-compound supports on KF catalyst and reaction kinetics of ethylene carbonate transesterification
IF 5.5
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2024-11-30
DOI:10.1016/j.jtice.2024.105832
引用次数: 0
Abstract
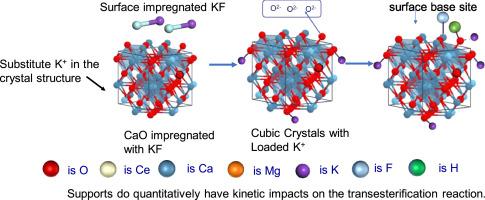
Heterogeneous catalysts can avoid the problem of difficult separation and recovery of homogeneous catalysts in transesterification reactions, but there is a lack of basis for process optimization because the quantitative influence mechanism of supports is not clear. In this work, we firstly attempt to make a comprehensive comparation on the effect of potassium fluoride (KF) catalysts on various supports (carbonates and oxides of cerium, magnesium and calcium) on the transesterification reaction, especially on the kinetics. According to the results, oxide-supported catalysts are significantly more active than carbonate-supported catalysts because they have more active sites. When oxides were used as supports, KF/MgO exhibited good activity because of its stronger basicity and higher number of active sites on the surface. Under the same process conditions, the stability time of KF/CeO2, KF/CaO and KF/MgO catalysed transesterification reaction was 240 min, 90 min and 30 min, respectively. KF/MgO achieved a conversion of 68.0 %. The activation energies of the KF/CeO2, KF/CaO and KF/MgO catalysed transesterification reaction were 22.22 kJ/mol, 15.93 kJ/mol and 15.80 kJ/mol, respectively. The surface alkalinity of KF/MgO increased the frequency of molecular collisions of the reactants and exhibited a faster reaction rate.
Ce/Ca/ mg复合载体对KF催化剂及碳酸乙烯酯交换反应动力学的影响
多相催化剂可以避免均相催化剂在酯交换反应中难以分离和回收的问题,但由于载体的定量影响机理尚不明确,缺乏工艺优化的依据。本文首先对不同载体(碳酸盐和铈、镁、钙的氧化物)上的氟化钾(KF)催化剂对酯交换反应的影响,特别是对动力学的影响进行了全面比较。结果表明,氧化物负载催化剂的活性明显高于碳酸盐负载催化剂,因为它们具有更多的活性位点。当使用氧化物作为载体时,KF/MgO具有较强的碱性和较多的表面活性位点,表现出良好的活性。在相同的工艺条件下,KF/CeO2、KF/CaO和KF/MgO催化酯交换反应的稳定时间分别为240 min、90 min和30 min。KF/MgO的转化率为68.0%。KF/CeO2、KF/CaO和KF/MgO催化的酯交换反应活化能分别为22.22 kJ/mol、15.93 kJ/mol和15.80 kJ/mol。KF/MgO的表面碱度增加了反应物的分子碰撞频率,反应速率加快。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


