Efficient photo-assisted reduction of Cr(VI) via activated carbon mediated Z-scheme FeS2/α-FeOOH/C heterojunction: Focusing on molecular oxygen fate and synergetic reduction mechanism
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
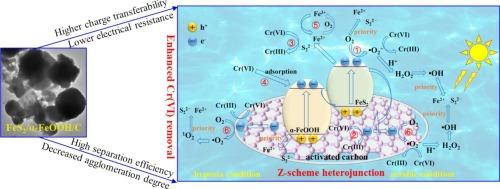
Herein, pyrite (FeS2), goethite (α-FeOOH) and activated carbon (C) were combined to construct FeS2/α-FeOOH/C composites with C mediated Z-scheme heterojunction for efficient photo-assisted Cr(VI) reduction. Characterization tests indicated the strong interaction via Fe-O-C and C-S-C, the increased amount of crystal defects and surface oxygenic functional groups, and the decreased agglomeration degree during combination strengthened electron transport and visible light conversion efficiency, resulting in synergistic effect for enhanced Cr(VI) removal. Additionally, C mediated Z-scheme heterojunction with lower resistance facilitated the transfer and separation efficiency of photo-generated carriers, further accelerating Cr(VI) reduction. Consequently, almost 100 % of Cr(VI) was reduced via FeS2/α-FeOOH/C in 60 min, 4 times that of pristine FeS2. Quenching, EPR and control experiments confirmed photocatalysis made more contribution than single reductive reagents (FeS2) in Cr(VI) reduction. The specific contribution rate of reductive species like e−, Fe2+, S22− and •O2− in this complicated system was firstly detailedly demonstrated and •O2− played a predominant role under oxic condition. Meanwhile, oxidative species like H2O2, 1O2 and •OH ascribed to DO activation or photo-generated h+ preferred to reacted with Fe2+ and S22− with higher reducibility and-only delayed Cr(VI) reduction rate. Hence, the final total Cr(VI) removal efficiency and generated Cr(III) kept stable in oxic solution.
活性炭介导的Z-scheme FeS2/α-FeOOH/C异质结光辅助Cr(VI)的高效还原研究:基于分子氧宿命和协同还原机制的研究
本文将黄铁矿(FeS2)、针铁矿(α-FeOOH)和活性炭(C)结合,构建了具有C介导z型异质结的FeS2/α-FeOOH/C复合材料,用于光辅助Cr(VI)的高效还原。表征实验表明,Fe-O-C和C-S-C的强相互作用,增加了晶体缺陷和表面含氧官能团的数量,结合过程中团聚程度的降低,增强了电子传递和可见光转换效率,形成了增强Cr(VI)去除的协同效应。此外,C介导的z型异质结具有较低的电阻,有利于光生载流子的转移和分离效率,进一步加速了Cr(VI)的还原。结果表明,FeS2/α-FeOOH/C在60 min内还原了几乎100%的Cr(VI),是原始FeS2的4倍。淬火、EPR和对照实验均证实光催化对Cr(VI)还原的贡献大于单一还原剂(FeS2)。本文首次详细论证了e−、Fe2+、S22−和•O2−等还原性物质在这一复杂体系中的具体贡献率,其中•O2−在氧化条件下起主导作用。与此同时,氧化物质如H2O2、1O2和•OH由于DO活化或光生h+倾向于与Fe2+和S22−反应,具有更高的还原性和延迟的Cr(VI)还原速率。因此,最终总Cr(VI)去除率和生成Cr(III)在氧溶液中保持稳定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


