Integrative greenness, blueness and whiteness determination of analytical RP-HPLC method for identifying Imipenem, Cilastatin and Relebactam in combined tablet form followed by forced degradation studies
引用次数: 0
Abstract
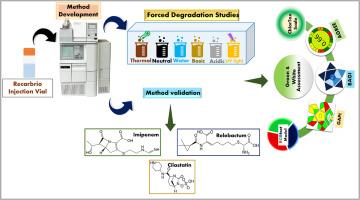
Most clinically preferred, US FDA and EU approved anti-infective drug, a multi-component formulation of Imipenem (IPM), Cilastatin (CS) and Relebactum (REL). The combination is used in medicating the most complicated, gram-negative bacterial infections. Henceforth, it requires the development of selective, precise, accurate, robust analytical method. Hence the present work comprises RP-HPLC method development for determination of IPM, CS and REL. The detection is achieved by using new and apt conditions i.e., Discovery C18 (4.6 × 150 mm, 5 µm) column, 0.01 N Potassium Dihydrogen Phosphate buffer: Acetonitrile (60:40) as mobile phase. The detection was found at short RT i.e., 2.32, 3.08, and 3.62-min for IPM, CS and REL correspondingly. Good sensitivity was observed, where LOD of 0.11 µg/ml (IPM), 0.14 µg/ml (CS), and 0.06 µg/ml (REL) was obtained. While, LOQ was noticed at 0.33 µg/ml (IPM), 0.43 µg/ml (CS) and 0.18 µg/ml (REL). As a counterpart, other study of forced degradation was also performed for the selected drugs at different extreme conditions. A sustainability evaluation has been done on the proposed method. The creative tools such as AGREE, GAPI, ChlorTox scale in green analytical counterpart were performed providing good score. Another BAGI software was opted as blue principle-evaluator, that resulted in a good score of 85 %. Highlighting the use of the most recent sustainability determination software, RGBfast model in present work with a good score of 74 %. Moreover, white and green evaluation has been performed in the form of comparative mode, for better understanding of the significance of current method.
亚胺培南、西司他汀和瑞巴坦联用片剂的绿、蓝、白综合测定及强制降解研究
临床首选,美国FDA和欧盟批准的抗感染药物,亚胺培南(IPM),西司他汀(CS)和瑞巴actum (REL)的多组分制剂。这种组合用于治疗最复杂的革兰氏阴性细菌感染。因此,需要发展有选择性的、精确的、准确的、稳健的分析方法。因此,本工作包括建立用于测定IPM, CS和REL的反相高效液相色谱方法。采用新的合适条件,即Discovery C18 (4.6 × 150 mm, 5µm)柱,0.01 N磷酸二氢钾缓冲液:乙腈(60:40)为流动相,实现检测。IPM、CS和REL的检测时间分别为2.32、3.08和3.62 min。该方法灵敏度高,检出限分别为0.11µg/ml (IPM)、0.14µg/ml (CS)和0.06µg/ml (REL)。定量限分别为0.33µg/ml (IPM)、0.43µg/ml (CS)和0.18µg/ml (REL)。同时,对所选药物在不同的极端条件下进行了强制降解的研究。对所提出的方法进行了可持续性评价。创新工具如AGREE、GAPI、ChlorTox标度等均在绿色分析对照品中获得良好的评分。选择另一款BAGI软件作为蓝色原则评估器,获得85%的高分。强调使用最新的可持续性确定软件,RGBfast模型在目前的工作中获得了74%的好分数。此外,为了更好地理解当前方法的意义,还以比较模式的形式进行了白色和绿色评价。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


