Halonal, an original benzoylated phenobarbital derivative anticonvulsant: in vivo evaluation, chemometric and molecular docking studies of enantiomers
IF 1.7
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
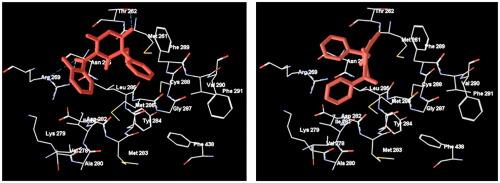
An original phenobarbital anticonvulsant Halonal, 5-ethyl-1-(2-fluorobenzoyl)-5-phenylpyrimidine-2,4,6(1H,3H,5H)-trione, stimulated the cellular immune and the humoral response in long-term alcoholized male (CBAxC57Bl/6) F1 mice to the level of healthy animals. Voltammetry was found to be suitable for determination of Halonal R/S-enantiomeric ratio, which was exemplified on the authentic sample with the R/S-composition of 40:60. Molecular docking (Schrödinger program, Glide) showed that Halonal behaved as a benzonal derivative interacting with GABAAR via the BARB binding site, with S-Halonal having higher similarity score than its R-enantiomer because of a different orientation of the 2-fluorobenzoyl substituent.
原苯甲酰苯巴比妥衍生物抗惊厥药:对映体的体内评价、化学计量学和分子对接研究
原苯巴比妥抗惊厥药Halonal, 5-乙基-1-(2-氟苯甲酰)-5-苯基嘧啶-2,4,6(1H,3H,5H)-三酮,刺激长期酒精化雄性(CBAxC57Bl/6) F1小鼠的细胞免疫和体液反应达到健康动物水平。伏安法适用于卤代酚对映体R/ s比的测定,并在R/ s组成为40:60的正品样品上进行了验证。分子对接(Schrödinger程序,Glide)表明,Halonal表现为通过BARB结合位点与GABAAR相互作用的苯并衍生物,由于2-氟苯甲酰取代基的取向不同,S-Halonal比r -对映体具有更高的相似性得分。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Mendeleev Communications
化学-化学综合
CiteScore
3.00
自引率
21.10%
发文量
226
审稿时长
4-8 weeks
期刊介绍:
Mendeleev Communications is the journal of the Russian Academy of Sciences, launched jointly by the Academy of Sciences of the USSR and the Royal Society of Chemistry (United Kingdom) in 1991. Starting from 1st January 2007, Elsevier is the new publishing partner of Mendeleev Communications.
Mendeleev Communications publishes short communications in chemistry. The journal primarily features papers from the Russian Federation and the other states of the former USSR. However, it also includes papers by authors from other parts of the world. Mendeleev Communications is not a translated journal, but instead is published directly in English. The International Editorial Board is composed of eminent scientists who provide advice on refereeing policy.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


