Application of a deep learning algorithm for the diagnosis of HCC
IF 9.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
Abstract
Background & Aims
Hepatocellular carcinoma (HCC) is characterized by a high mortality rate. The Liver Imaging Reporting and Data System (LI-RADS) results in a considerable number of indeterminate observations, rendering an accurate diagnosis difficult.
Methods
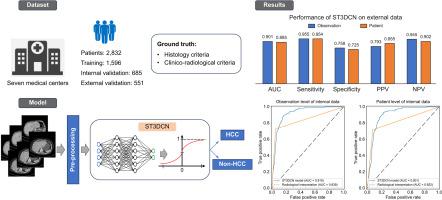
We developed four deep learning models for diagnosing HCC on computed tomography (CT) via a training–validation–testing approach. Thin-slice triphasic CT liver images and relevant clinical information were collected and processed for deep learning. HCC was diagnosed and verified via a 12-month clinical composite reference standard. CT observations among at-risk patients were annotated using LI-RADS. Diagnostic performance was assessed by internal validation and independent external testing. We conducted sensitivity analyses of different subgroups, deep learning explainability evaluation, and misclassification analysis.
Results
From 2,832 patients and 4,305 CT observations, the best-performing model was Spatio-Temporal 3D Convolution Network (ST3DCN), achieving area under receiver-operating-characteristic curves (AUCs) of 0.919 (95% CI, 0.903–0.935) and 0.901 (95% CI, 0.879–0.924) at the observation (n = 1,077) and patient (n = 685) levels, respectively during internal validation, compared with 0.839 (95% CI, 0.814–0.864) and 0.822 (95% CI, 0.790–0.853), respectively for standard of care radiological interpretation. The negative predictive values of ST3DCN were 0.966 (95% CI, 0.954–0.979) and 0.951 (95% CI, 0.931–0.971), respectively. The observation-level AUCs among at-risk patients, 2–5-cm observations, and singular portovenous phase analysis of ST3DCN were 0.899 (95% CI, 0.874–0.924), 0.872 (95% CI, 0.838–0.909) and 0.912 (95% CI, 0.895–0.929), respectively. In external testing (551/717 patients/observations), the AUC of ST3DCN was 0.901 (95% CI, 0.877–0.924), which was non-inferior to radiological interpretation (AUC 0.900; 95% CI, 0.877–-923).
Conclusions
ST3DCN achieved strong, robust performance for accurate HCC diagnosis on CT. Thus, deep learning can expedite and improve the process of diagnosing HCC.
Impact and implications:
The clinical applicability of deep learning in HCC diagnosis is potentially huge, especially considering the expected increase in the incidence and mortality of HCC worldwide. Early diagnosis through deep learning can lead to earlier definitive management, particularly for at-risk patients. The model can be broadly deployed for patients undergoing a triphasic contrast CT scan of the liver to reduce the currently high mortality rate of HCC.
一种深度学习算法在HCC诊断中的应用
背景,目的肝细胞癌(HCC)的特点是死亡率高。肝脏成像报告和数据系统(LI-RADS)导致大量不确定的观察结果,使准确诊断变得困难。方法采用训练-验证-测试的方法,建立了4种用于CT诊断HCC的深度学习模型。收集肝脏薄层三相CT图像及相关临床信息进行深度学习处理。通过12个月的临床综合参考标准诊断和证实HCC。高危患者的CT观察使用LI-RADS进行注释。通过内部验证和独立的外部测试来评估诊断性能。我们进行了不同亚组的敏感性分析、深度学习可解释性评估和错误分类分析。结果在2,832例患者和4,305例CT观察中,表现最好的模型是时空三维卷积网络(ST3DCN),在内部验证时,在观察(n = 1,077)和患者(n = 685)水平上,接受者操作特征曲线下面积(auc)分别为0.919 (95% CI, 0.903-0.935)和0.901 (95% CI, 0.879-0.924),而在内部验证时,前者为0.839 (95% CI, 0.814-0.864)和0.822 (95% CI, 0.790-0.853)。分别为护理标准放射学解释。ST3DCN阴性预测值分别为0.966 (95% CI, 0.954-0.979)和0.951 (95% CI, 0.931-0.971)。高危患者、2 - 5 cm观察和ST3DCN单门静脉相分析的观察水平auc分别为0.899 (95% CI, 0.874-0.924)、0.872 (95% CI, 0.838-0.909)和0.912 (95% CI, 0.895-0.929)。在外部检测(551/717例患者/观察)中,ST3DCN的AUC为0.901 (95% CI, 0.877-0.924),不低于放射学解释(AUC 0.900;95% ci, 0.877—923)。结论st3dcn对肝癌的CT准确诊断具有较强的稳健性。因此,深度学习可以加快和改善HCC的诊断过程。影响和启示:深度学习在HCC诊断中的临床应用潜力巨大,特别是考虑到全球HCC发病率和死亡率的预期增长。通过深度学习进行早期诊断可以实现更早的明确管理,特别是对高危患者。该模型可广泛应用于肝脏CT三相对比扫描患者,以降低目前HCC的高死亡率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


