Recognition of carboxylic acids and phosphonic acids using a 1,8-diphenylanthracene-based diguanidine in solution and solid states
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A 1,8-diphenylanthracene-based diguanidine 1 has been designed and synthesized for the recognition of carboxylic acids and phosphonic acid derivatives. The diguanidine 1a forms 1:1 complexes with the dicarboxylic acids and the diphosphonic acid derivatives in a DMSO solution, and the formation of these complexes was confirmed by a DOSY NMR analysis. The binding mode towards the guanidyl group (Type I, Type II, two existing types of binding modes toward three nitrogen atoms) with a diphosphonic acid derivative was successfully demonstrated by the 2D NOESY analysis. The diguanidine 1a does not show any fluorescence in a DMSO solution, but after the addition of carboxylic acids, the formation of a stable complex or the formation of the protonated guanidinium (1a+2H+) were distinguishable by the observation of a light-blue fluorescence around 430 nm. These fluorescence observations can be determined the stability of the complexes of the diguanidine 1a with carboxylic acids. On the other hand, the DMSO solution of diguanidine 1a showed a light-blue fluorescence after the addition of phosphonic acids. Interestingly, diguanidine 1a recognized methylphosphonic acid 9a in the solid state by grinding and showed a green fluorescence, while the other phosphonic acids 9 and dicarboxylic acids (4d: pimelic acid, 4f: azelaic acid) showed a light-blue fluorescence in the solid state, and a selectivity was observed. These fluorescence characteristics of the diguanidine 1 are applicable for the detection of carboxylic acids and phosphonic acids, especially for methylphosphonic acid 9a in the solid state.
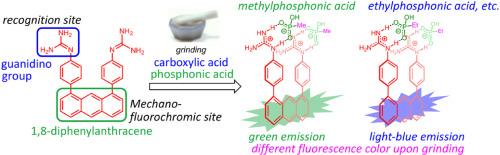
用1,8-二苯基二苯胺在溶液和固体状态下识别羧酸和膦酸
设计并合成了1,8-二苯基二苯胺1,用于识别羧酸和膦酸衍生物。二胍1a在DMSO溶液中与二羧酸和二膦酸衍生物形成1:1的配合物,并通过DOSY NMR分析证实了这些配合物的形成。通过二维NOESY分析,成功地证明了二膦酸衍生物与胍基的结合模式(I型、II型,两种现有的与三个氮原子的结合模式)。双胍1a在DMSO溶液中不显示任何荧光,但在加入羧酸后,稳定络合物的形成或质子化胍(1a+2H+)的形成可以通过观察430 nm左右的浅蓝色荧光来区分。这些荧光观察可以确定二胍1a与羧酸配合物的稳定性。另一方面,加入膦酸后,双胍1a的DMSO溶液呈现浅蓝色荧光。有趣的是,二胍1a通过研磨识别固体状态下的甲基膦酸9a并发出绿色荧光,而其他膦酸9和二羧酸(4d:戊二酸,4f:壬二酸)在固体状态下显示浅蓝色荧光,并观察到选择性。二胍1的这些荧光特性适用于羧酸和膦酸的检测,尤其适用于固态甲基膦酸9a的检测。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


