Solvent effect on facile in situ precipitation of nickel–iron hydroxide for enhanced overall water splitting
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
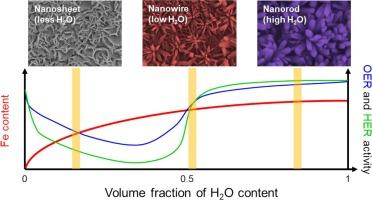
Efficient and economical electrocatalysts are essential for addressing the high overpotential challenges of the oxygen evolution reaction (OER) in electrochemical water splitting. This study explores the synthesis of nickel–iron hydroxide (NiFeOH) catalysts via in situ precipitation, focusing on the impact of the solvent composition on the morphology and catalytic performance of the material. The volumetric ratio of H2O to ethanol in the solvent mixture was systematically varied, revealing that higher proportions of H2O promoted the formation of thicker and larger needle-like NiFeOH structures. In contrast with the common preference for thin needle-shaped morphologies, our findings reveal that these thicker structures exhibit superior electrocatalytic activity. This enhanced performance is attributed to the higher iron content and faster reaction kinetics promoted by the increased permittivity of water-rich environments. The optimized NiFeOH catalysts, particularly those with higher water content, exhibit excellent OER and hydrogen evolution reaction (HER) activities, achieving low overpotentials of 288 mV at 100 mA cm−2 for OER and 131 mV at 10 mA cm−2 for HER. Furthermore, long-term stability tests confirmed the robustness of the catalysts, with minimal morphological degradation and consistent performance in overall water splitting. This work highlights the significance of solvent effects in tailoring the morphology and catalytic properties of NiFeOH, providing valuable insights for the design of effective water-splitting electrocatalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


