Hydrogen behavior in Ti surface nitridation: Hydrogen as an active component catalyzes the nitridation reaction
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Typically, H2 is introduced into the nitriding atmosphere to enhance the efficiency of metal surface nitridation. However, the role of hydrogen as an active species in catalyzing the reaction is still uncertain. Here, we present the nitridation mechanism of pure titanium(Ti) surface (hexagonal, α phase) with and without H atoms(H) based on first-principles calculations of surface nitrogen adsorption, dissociation and penetration. First, the adsorption characteristics of N atoms(N) and N2 molecules(N2) and the system energy indicate that FCC and HCP are favorable adsorption sites. In contrast, the adsorption stability of N on H-containing surfaces shows higher trend. Second, the minimum energy path (MEP) study results based on the climb image nudged elastic band (CI-NEB) method show that H increases the efficiency of dissociation and penetration processes. A linear relationship exists between hydrogen concentration and the N penetration energy barrier. In addition, electronic structure calculations theoretically support H regulation of surface nitridation efficiency. The nearby H reduces the energy barrier for the dissociation of N2 and the penetration of N by enhancing the interaction between N and surrounding Ti. This study provides theoretical support and new insights into the microscopic mechanism of H regulating the nitridation reaction of the Ti surface.
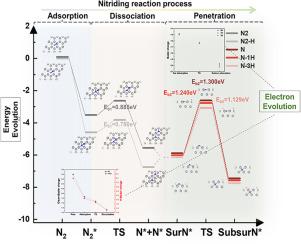
氢在钛表面氮化中的行为:氢作为活性组分催化氮化反应
通常,在氮化气氛中引入H2来提高金属表面氮化的效率。然而,氢作为活性物质在催化反应中的作用仍不确定。本文基于表面氮吸附、解离和渗透的第一性原理计算,研究了纯钛(Ti)表面(六方,α相)在含和不含H原子(H)的情况下的氮化机理。首先,对N原子(N)和N2分子(N2)的吸附特性和系统能表明FCC和HCP是有利的吸附位点。相比之下,氮在含h表面的吸附稳定性呈现出更高的趋势。其次,基于爬升图像微推弹性带(CI-NEB)方法的最小能量路径(MEP)研究结果表明,H提高了解离和穿透过程的效率。氢浓度与N穿透能垒之间存在线性关系。此外,电子结构计算在理论上支持表面氮化效率的H调节。附近的H通过增强N与周围Ti的相互作用,降低了N2解离和N穿透的能垒。本研究为H调控Ti表面氮化反应的微观机理提供了理论支持和新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


