Scalable fabrication of freely shapable 3D hierarchical Cu-doped hydroxyapatite scaffolds via rapid gelation for enhanced bone repair
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Critical-sized bone defects present a formidable challenge in tissue engineering, necessitating innovative approaches that integrate osteogenesis and angiogenesis for effective repair. Inspired by the hierarchical porous structure of natural bone, this study introduces a novel method for the scalable production of ultra-long, copper-doped hydroxyapatite (Cu-HAp) fibers, utilizing the rapid gelation properties of guar gum (GG) under controlled conditions. These fibers serve as foundational units to fabricate three-dimensional porous scaffolds with a biomimetic hierarchical architecture. The scaffolds exhibit a broad pore size distribution (1–500 μm) and abundant nanoporous features, mimicking the native bone extracellular matrix. Physicochemical characterization and in vitro assays demonstrated that the copper doping significantly enhanced osteogenic and angiogenic activities, with optimized concentrations (0.8 % and 1.2 % Cu) facilitating the upregulation of osteogenesis-related genes and proteins, as well as promoting endothelial cell proliferation. In vivo studies further confirmed the scaffolds' efficacy, with the 1.2Cu-HAp group showing a remarkable increase in bone regeneration (bone volume/total volume ratio: 35.7 ± 1.87 %) within the defect site. This research offers a promising strategy for the rapid fabrication of multifunctional scaffolds that not only support bone tissue repair but also actively accelerate the healing process through enhanced vascularization.
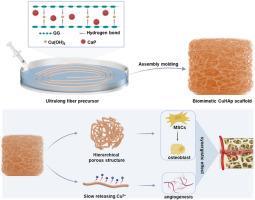
快速凝胶法制备可自由成形的三维分层掺铜羟基磷灰石支架,增强骨修复
临界尺寸的骨缺损在组织工程中提出了一个巨大的挑战,需要创新的方法来整合成骨和血管生成来有效修复。受天然骨的分层多孔结构的启发,本研究介绍了一种在受控条件下利用瓜尔胶(GG)的快速凝胶特性可扩展生产超长铜掺杂羟基磷灰石(Cu-HAp)纤维的新方法。这些纤维作为基础单位,以制造具有仿生层次结构的三维多孔支架。该支架具有较宽的孔径分布(1 ~ 500 μm)和丰富的纳米孔特征,模拟了天然骨细胞外基质。理化表征和体外实验表明,铜掺杂显著增强了成骨和血管生成活性,优化浓度(0.8%和1.2% Cu)促进了成骨相关基因和蛋白质的上调,并促进了内皮细胞的增殖。体内实验进一步证实了支架的有效性,1.2Cu-HAp组缺损部位骨再生显著增加(骨体积/总体积比:35.7±1.87%)。该研究为快速制造多功能支架提供了一种有希望的策略,该支架不仅支持骨组织修复,而且通过增强血管化积极加速愈合过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


