A three-phase strategy of bionic drug reservoir scaffold by 3D printing and layer-by-layer modification for chronic relapse management in traumatic osteomyelitis
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
We have developed a novel three-phase strategy for osteomyelitis treatment, structured into three distinct phases: the “strong antimicrobial” phase, the “monitoring and osteogenesis” phase and the “bone repair” phase. To implement this staged therapeutic strategy, we engineered a bionic drug reservoir scaffold carrying a dual-drug combination of antimicrobial peptides (AMPs) and simvastatin (SV). The scaffold integrated a bilayer gel drug-carrying structure, based on an induced membrane and combined with a 3D-printed rigid bone graft using a layer-by-layer modification strategy. The mechanical strength of the composite scaffold (73.40 ± 22.44 MPa) is comparable to that of cancellous bone. This scaffold enables controlled, sequential drug release through a spatial structure design and nanoparticle drug-carrying strategy. AMPs are released rapidly, with the release efficiency of 74.90 ± 8.19 % at 14 days (pH = 7.2), thus enabling rapid antimicrobial therapy. Meanwhile, SV is released over a prolonged period, with a release efficiency of 98.98 ± 0.05 % over 40 days in vitro simulations, promoting sustained osteogenesis and facilitating the treatment of intracellular infections by activating macrophage extracellular traps (METs). The antimicrobial, osteogenic and immunomodulatory effects of the scaffolds were verified through in vitro and in vivo experiments. It was demonstrated that composite scaffolds were able to combat the chronic recurrence of osteomyelitis after debridement, by providing rapid sterilization, stimulating METs formation, and supporting osteogenic repair.
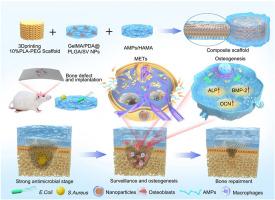
外伤性骨髓炎慢性复发治疗的3D打印及逐层修饰仿生药物储存库支架三相策略
我们已经开发了一种新的骨髓炎治疗的三阶段策略,分为三个不同的阶段:“强抗菌”阶段,“监测和成骨”阶段和“骨修复”阶段。为了实现这种分阶段治疗策略,我们设计了一个仿生药物储存库支架,携带抗菌肽(amp)和辛伐他汀(SV)的双药组合。该支架集成了基于诱导膜的双层凝胶载药结构,并使用逐层修饰策略将其与3d打印的刚性骨移植物相结合。复合支架的机械强度(73.40±22.44 MPa)与松质骨相当。这种支架通过空间结构设计和纳米颗粒载药策略实现可控的、顺序的药物释放。amp释放迅速,在14天(pH = 7.2)释放效率为74.90±8.19%,从而实现快速抗菌治疗。同时,SV的释放时间较长,体外模拟40天的释放效率为98.98±0.05%,通过激活巨噬细胞胞外陷阱(METs)促进持续成骨,促进细胞内感染的治疗。通过体外和体内实验验证了支架的抗菌、成骨和免疫调节作用。研究表明,复合支架能够通过提供快速灭菌、刺激METs形成和支持成骨修复来对抗清创后骨髓炎的慢性复发。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


