Improved carboxylate density hydrochar by alkylation of surface phenol for adsorption of cationic dye in aqueous solution
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Methylene blue (MB) adsorption in aqueous solution studied using rice straw derived hydrochar (RSH) and carboxylate functionalized hydrochar (RSHC) as adsorbent. RSHC with improved carboxylate functional group density was prepared by selective functionalization of phenolic oxygen functional groups present on RSH surface. The RSH and RSHC were characterized by ultimate and proximate analysis, Boehm Titration, 13C CP-MAS SS NMR, XPS, SEM, BET surface area and zeta potential analysis. Batch adsorption experiments were optimized for MB adsorption were, adsorbent dose of 0.2 g/L, pH 8 and 7, respectively for RSH and RSHC, with 3.5 h contact time at 298 K. Nonlinear fit to Langmuir, Freundlich, Temkin, d-R models and Sips isotherm models, showed good fit with R2>0.95. RSHC (Qm = 434.6 mg/g) showed four-fold increase in MB adsorption as compared to the pristine hydrochar RSH (Qm =111.6 mg/g). Contact time study showed good nonlinear fit to pseudosecond order model indicating a strong interaction between hydrochar adsorbents and MB. Thermodynamics of MB adsorption (288–308 K) onto the surface of RSH and RSHC, is a spontaneous process with ∆G° -23.85 and -28.31 kJ/mol, respectively. RSH in characterized by enthalpy driven adsorption characteristic of proton-MB exchange, whereas in case of RSHC, K+–MB exchange is entropically driven process.
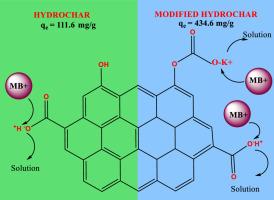
用表面苯酚烷基化提高羧酸盐密度的碳氢化合物在水溶液中对阳离子染料的吸附
研究了以稻秆衍生氢炭(RSH)和羧酸盐功能化氢炭(RSHC)为吸附剂对水溶液中亚甲基蓝(MB)的吸附作用。通过对RSH表面的酚氧官能团进行选择性官能团化,制备了羧酸盐官能团密度较高的RSHC。通过极限和近似分析、Boehm滴定、13C CP-MAS - SS NMR、XPS、SEM、BET表面积和zeta电位分析对RSH和RSHC进行了表征。批量吸附实验优化的MB吸附条件为:吸附剂用量为0.2 g/L, pH为8和7,分别为RSH和RSHC,接触时间为3.5 h,温度为298 K。对Langmuir、Freundlich、Temkin、d-R模型和Sips等温模型进行非线性拟合,拟合系数为R2>;0.95。RSHC (Qm = 434.6 mg/g)对MB的吸附比原始烃类RSH (Qm =111.6 mg/g)提高了4倍。接触时间研究结果表明,烃类吸附剂与MB之间存在较强的相互作用。吸附过程(288 ~ 308 K)为自发过程,吸附量分别为∆G°-23.85和-28.31 kJ/mol。RSH表现为质子-MB交换的焓驱动吸附特性,而RSHC则表现为K+ -MB交换的熵驱动过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


