Improving photocatalytic performance of nickel oxide infused chitosan nanocomposite for adsorption of organic dyes
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
One of the most significant issues worldwide is access to safe drinking water for human consumption, which has necessitated high intensification efforts from scientists to come up with a lasting solution. Nanotechnology-treated water contains no bacteria and organic dyes and is deemed superior to such methods of treatment. In this paper, an investigation into the light-induced effect on chitosan nanoparticles containing biosynthesized NiO nanoparticles was carried out. The synthesized nanocomposites have utilized characterizations using several methods, like FETEM, FTIR, XRD, XPS, and UV-visible spectroscopy. Under this work, the synthesis and characterization of the NiO@CS nanocomposite, used as an adsorbent to remove organic dyes from water. The organic dyes used were, among others, Congo red (CR) and Malachite green oxalate (MGO). The effect of temperature, pH, dosage, pH, and time on the removal efficiency (%) of adsorbent towards various colours was separately investigated. Kinetics isotherms Langmuir, Freundlich and Temkin as well as thermodynamic parameters (ΔG°, ΔH° and ΔS°) were observed. The linear correlation coefficient, R2 of the Langmuir adsorption isotherm was almost equal to 1 for all colors which suggests that the adsorption occurred as a monolayer. Adsorption occurred with a reduction in negative enthalpy, free energy, and a drop in entropy at 30°C. The adsorbent was reusable for three cycles without a reduction in removal efficiency (%). Therefore, the NiO@CS nanocomposite may be feasibly applied toward commercial applications from organic colors from aqueous solutions.
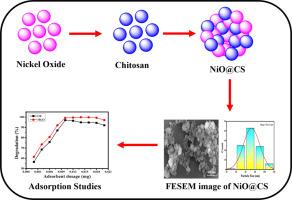
改善氧化镍注入壳聚糖纳米复合材料对有机染料的光催化吸附性能
世界范围内最重要的问题之一是获得供人类消费的安全饮用水,这需要科学家们高度加强努力,以提出一个持久的解决方案。纳米技术处理过的水不含细菌和有机染料,被认为优于这些处理方法。本文研究了含生物合成纳米镍的壳聚糖纳米颗粒的光致效应。合成的纳米复合材料使用多种方法进行了表征,如FETEM, FTIR, XRD, XPS和uv -可见光谱。本文研究了NiO@CS纳米复合材料的合成和表征,并将其作为吸附水中有机染料的吸附剂。所使用的有机染料包括刚果红(CR)和草酸孔雀石绿(MGO)。分别考察了温度、pH、投加量、pH和时间对吸附剂对各种颜色去除率的影响。观察了动力学等温线Langmuir, Freundlich和Temkin以及热力学参数(ΔG°,ΔH°和ΔS°)。Langmuir吸附等温线的线性相关系数R2几乎等于1,表明吸附是单层的。在30°C时,吸附随负焓、自由能和熵的降低而发生。吸附剂可重复使用三次,去除率(%)未降低。因此,NiO@CS纳米复合材料可能可行地应用于从水溶液中提取有机色素的商业应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


