Optimization and Scale-Up Synthesis of a Lappaconitine Alkaloid Derivative, QG3030, as a Novel Osteoanabolic Agent
IF 3.1
3区 化学
Q2 CHEMISTRY, APPLIED
引用次数: 0
Abstract
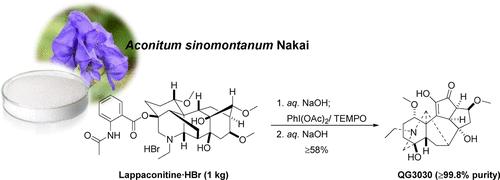
Our previous work revealed that the novel lappaconitine alkaloid derivative, QG3030 (6), has an enhanced osteogenesis effect in the ovariectomized rat model without acute oral toxicity. QG3030 (6) recently received approval for the Investigational New Drug application for its osteoporosis treatment from the Korean Ministry of Food and Drug Safety. Therefore, the need for an economical, large-scale production of QG3030 (6) motivated the development of a novel synthetic procedure for its clinical studies. We herein report an efficient, safe, and cost-effective synthesis of QG3030 (6) as a clinical candidate for osteoporosis treatment. As an optimized synthetic procedure, the reaction of lappaconitine·HBr (1·HBr, 1.0 kg scale) with co-oxidizing agents PhI(OAc)2-TEMPO (1.5 and 2 equiv) as a key step in a mixed EtOAc-acetone solution (v/v = 2/1) furnished α,β-unsaturated ketone (4), which was then treated with aq. NaOH to provide pure QG3030 (6, 352 g) in 58% overall yield with a purity of 99.8% after crystallization from EtOH–CH2Cl2. This pilot synthetic procedure was performed three times, and the reproducible results were obtained with both nearly identical yields and purities.
新型骨合成代谢剂高乌藤碱生物碱衍生物QG3030的优化与规模化合成
我们之前的研究表明,新型高穿甲碱生物碱衍生物QG3030(6)在去卵巢大鼠模型中具有增强成骨作用,且无急性口服毒性。QG3030(6)最近获得了韩国食品和药物安全部批准的用于治疗骨质疏松症的研究新药申请。因此,对经济、大规模生产QG3030(6)的需求推动了一种用于其临床研究的新型合成工艺的发展。我们在此报道了一种高效、安全且具有成本效益的QG3030合成方法(6),作为骨质疏松症治疗的临床候选药物。优化后的合成工艺为:以1·HBr(1·HBr, 1.0 kg)与共氧化剂PhI(OAc)2- tempo(1.5和2当量)为关键步骤,在混合的etoac -丙酮溶液(v/v = 2/1)中生成α,β-不饱和酮(4),然后用aq. NaOH处理,从EtOH-CH2Cl2结晶后,得到纯度为6,352 g的QG3030,总收率为58%,纯度为99.8%。该中试合成过程进行了三次,获得了几乎相同收率和纯度的可重复性结果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.90
自引率
14.70%
发文量
251
审稿时长
2 months
期刊介绍:
The journal Organic Process Research & Development serves as a communication tool between industrial chemists and chemists working in universities and research institutes. As such, it reports original work from the broad field of industrial process chemistry but also presents academic results that are relevant, or potentially relevant, to industrial applications. Process chemistry is the science that enables the safe, environmentally benign and ultimately economical manufacturing of organic compounds that are required in larger amounts to help address the needs of society. Consequently, the Journal encompasses every aspect of organic chemistry, including all aspects of catalysis, synthetic methodology development and synthetic strategy exploration, but also includes aspects from analytical and solid-state chemistry and chemical engineering, such as work-up tools,process safety, or flow-chemistry. The goal of development and optimization of chemical reactions and processes is their transfer to a larger scale; original work describing such studies and the actual implementation on scale is highly relevant to the journal. However, studies on new developments from either industry, research institutes or academia that have not yet been demonstrated on scale, but where an industrial utility can be expected and where the study has addressed important prerequisites for a scale-up and has given confidence into the reliability and practicality of the chemistry, also serve the mission of OPR&D as a communication tool between the different contributors to the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


