Habitat edges decrease plant reproductive output in fragmented landscapes
IF 5.3
1区 环境科学与生态学
Q1 ECOLOGY
引用次数: 0
Abstract

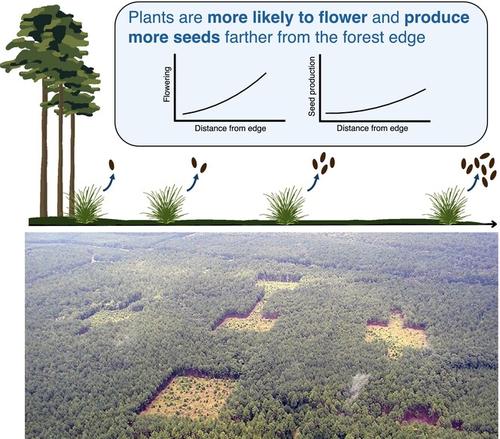
在破碎景观中,生境边缘降低了植物的生殖输出
栖息地丧失是生物多样性的主要威胁(Dirzo et al., 2014;Newbold et al., 2015;Tilman et al., 2017)。虽然栖息地丧失对生物多样性的负面影响是明确的,但关于栖息地破碎化的影响存在更多的争论,这往往与栖息地丧失相混淆(Fahrig, 2017;Fahrig等人,2019;Fletcher et al., 2018;Haddad et al., 2015)。为了解决这一争论,研究生物多样性变化的机制,如物种内部的人口统计过程,可能会澄清破碎景观中的生物多样性趋势(Fletcher et al., 2023;Pardini et al., 2017)。种群人口统计决定了物种的持久性,尤其是小种群,多物种的累积响应可能导致群落水平的生物多样性变化(Paniw et al., 2023;Schmidt et al., 2022)。过去的人口碎片化研究主要集中在移民和移民的过程上(Honnay et al., 2005;Jacquemyn et al., 2002)。然而,其他人口过程,如生殖成功率,也可能受到碎片化的影响(Aguilar等人,2019)。鉴于生殖是人口增长的一个组成部分(Koons et al., 2017),碎片化对生殖产出的影响可能对人口持久性产生重要影响。由于碎片化导致了在多个空间尺度上出现的几种空间格局(Fletcher等人,2023),能够分离出这些空间格局影响的实验是有价值的。例如,当一定数量的栖息地被分割时,在景观尺度上,栖息地斑块的数量会增加,这降低了斑块间尺度上栖息地结构的连通性(Fletcher et al., 2023)。同时,破碎化的栖息地也会产生更多的边缘栖息地,增加斑块和景观尺度上的边缘面积比,减少斑块内尺度上到边缘的平均距离(Fletcher et al., 2023)。这些破碎化的多个组成部分可能通过影响授粉、生长、种子捕食或食草性来影响植物的生殖输出(即种子生产)(Brudvig等人,2015)。然而,尽管人们普遍认识到栖息地丧失和破碎化的影响经常被混淆(Ewers &;杜顿,2005;Fahrig, 2003;Valente et al., 2023),解开它们的影响仍然具有挑战性。以前对植物生殖输出的研究通常集中在斑块大小上,以测试碎片化效应(Bruna &;克雷斯,2002;Portela et al., 2021;Tomimatsu,Ohara, 2010),将破碎化的多个组成部分与栖息地丧失相混淆。将破碎化的多个组成部分的影响从栖息地丧失中分离出来的实验将阐明破碎化地区人口统计学变化的机制,正如我们在这里使用的实验破碎化系统。栖息地破碎化会在孤立的斑块中造成不连贯的种群,这可能会通过破坏花粉运动来减少植物的生殖产出(Betts等人,2019)。授粉是绝大多数植物物种繁殖成功的关键过程(Friedman &;巴雷特,2009;Ollerton et al., 2011),这意味着景观变化对授粉的破坏会对植物的生殖输出产生负面影响。破碎化导致的种群空间隔离可能会减少花粉运动(Hadley &;Betts, 2012),随后减少基因流动,导致近亲繁殖的可能性更高(Aguilar等人,2019;Rosas et al., 2011)。风传粉和昆虫传粉都可能因破碎化而减少,但减少的机制不同。依赖于植物-传粉者共生关系的物种的授粉与其传粉者的碎片化效应直接相关,花粉运动与传粉者的响应相对应(Kormann et al., 2016)。小块间的连通性促进了传粉者的移动(Tewksbury等人,2002),增加了昆虫传粉物种的花粉移动(Townsend &;利维,2005)。然而,对于风媒传粉的物种来说,破碎化所产生的非生物条件,如边缘和隔离的增加,可能是通过改变风动力来限制授粉的原因(Aguilar等人,2019;Damschen et al., 2014)。开放生境的结构连通性增加了斑块之间的风运动,特别是当与主导风对齐时(Damschen et al., 2014),这可能促进花粉在离散种群之间的运动(Provan et al., 2008)。然而,由于物种对破碎化反应的差异(Ewers &;杜顿,2005;费舍尔,Lindenmayer, 2007),需要更多的工作来了解传粉模式是否在传粉模式之间是一致的,以及解开多个碎片化成分对传粉的影响,这些影响可能混淆碎片化效应(Brudvig等)。 , 2015;喜力啤酒,韦伯,2013;Newman et al., 2013)。虽然花粉运动通常是在碎裂的背景下考虑的,但碎裂也可能通过开花和物候的种群水平变化影响植物的生殖输出。边缘栖息地通常拥有独特的小气候条件,不断变化的非生物条件,如温度、湿度和光照可用性(Tuff et al., 2016)。由于植物的生长和开花高度取决于非生物条件,这些非生物变化可能影响植物的开花和种子生产(Galloway &;伯吉斯,2012;m<s:1> ller等人,2021;Suzán-Azpiri等人,2017)。此外,植物适合度可以通过昆虫访虫的边缘效应间接影响。传粉媒介和食草昆虫可能受到非生物边缘条件的影响,进一步影响种子结实和植物生长(Andrieu et al., 2018;Levey et al., 2016;Ren et al., 2023)。由于人口结构(例如开花个体的比例)和生殖产出可以促进种群增长(Caughlin等人,2019),植物开花和种子生产的边缘效应可能会影响植物种群动态(Bruna &;克雷斯,2002;Suzán-Azpiri等人,2017)。植物种群的增长是由几个人口统计学速率决定的,包括繁殖力、建立、存活和生长。Hone, 2002),它们都可能受到栖息地破碎化的影响(Bruna &;奥利,2005;Honnay et al., 2005)。然而,这些人口比率对种群动态的相对重要性可能因物种的生活史、当地非生物环境和生物相互作用等因素而异(Crone, 2001;de Kroon et al., 1986)。因此,如果一个物种的种子有限,种子生产可能对种群的增长和持久性非常重要,但如果栖息地条件限制了生存或生长,则不那么重要(Clark et al, 2007)。在我们的长叶松稀树草原栖息地实验系统中,之前的工作发现,对于两个长寿的多年生物种,种子产量是预测种群增长的最重要的人口统计学参数(Caughlin et al., 2019)。然而,对于早期演代物种来说,微场地条件和种子捕食比种子丰度更重要(Orrock等,2006),这突出了即使在一个系统内,人口驱动因素的可变性。总体而言,尽管种子产量对植物种群持久性的相对重要性可能有所不同,但测量生殖产量可以深入了解人口统计学的一个组成部分如何受到景观变化的影响(Bruna &;克雷斯,2002;Caughlin等人,2019;Suzán-Azpiri等人,2017)。在这里,我们测试碎片化如何影响植物的生殖输出,看看碎片化对植物开花、授粉和种子生产的影响。我们进行了一项大规模的重复碎片化实验,旨在控制碎片化的三个方面:斑块之间的连通性、斑块尺度的边缘面积比和斑块内与边缘的距离。我们实验种植了三种风媒传粉和两种虫媒传粉的植物,以探讨(1)连通性、边面积比和与边缘的距离是否影响植物开花的可能性和花的丰度?(2)如果植物开花,连通性、边面积比和到边的距离是否会影响传粉率和种子产量?我们预计在栖息地边缘附近和未连通的斑块中植物的生殖产量(种子产量)会减少。具体而言,在我们的开放式生境斑块和森林基质系统中,我们预计树冠边缘遮荫的非生物效应会减少开花,降低边缘附近植物的繁殖产量。此外,我们预计由于风传粉和昆虫传粉物种的花粉运动中断,在不相连的斑块中传粉会减少。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Ecology
环境科学-生态学
CiteScore
10.90
自引率
5.50%
发文量
207
审稿时长
3.0 months
期刊介绍:
Journal of Ecology publishes original research papers on all aspects of the ecology of plants (including algae), in both aquatic and terrestrial ecosystems. We do not publish papers concerned solely with cultivated plants and agricultural ecosystems. Studies of plant communities, populations or individual species are accepted, as well as studies of the interactions between plants and animals, fungi or bacteria, providing they focus on the ecology of the plants.
We aim to bring important work using any ecological approach (including molecular techniques) to a wide international audience and therefore only publish papers with strong and ecological messages that advance our understanding of ecological principles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


