Strengthened High-Concentration Quasi-Solid Electrolytes for Lithium Metal with Ultralong Stable Cyclability
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The lithium metal batteries coupled with nickel-rich LiNixCoyMn1–x–yO2 (x > 0.7) cathodes hold promise for surpassing the current energy density limit of lithium-ion batteries. However, conventional electrolytes containing free active solvents are highly susceptible to decomposition, particularly at the interfaces of lithium anode and high-voltage cathode. Herein, we have developed a composite quasi-solid electrolyte (CQSE) utilizing sulfated Al2O3 (S-Al2O3)-bridged cellulose triacetate (CTA) to stabilize the interfaces between the electrolyte and electrodes. S-Al2O3 competitively dissociates Li+ through coordination interactions with anions, facilitating the formation of a distinctive solvation structure characterized by prevalent ion pairs and aggregates. In addition, coordination of S-Al2O3 with CTA forms S-Al2O3-bridged CTA molecular chain networks, enhancing the mechanical strength of the CQSE and immobilizing free liquid molecules. Consequently, the CQSE demonstrates an enhanced tensile strength of up to 7.4 MPa and a high ionic conductivity of 1.8 × 10–3 S cm–1 at room temperature. Furthermore, the CQSE not only suppresses electrode–electrolyte side reactions but also enables the formation of an inorganic-rich solid/cathode electrolyte interphase. As a result, the Li|CQSE|LiNi0.83Co0.11Mn0.06O2 (NCM83) batteries retain 84% capacity after 1000 cycles at 1 C, with the pouch cells demonstrating 80% capacity retention after 250 cycles at 0.5 C.
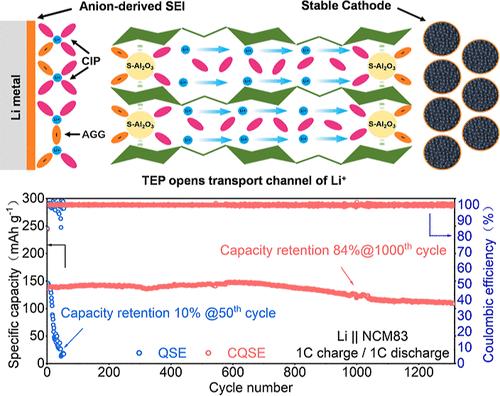
具有超长稳定循环性能的强化高浓度准固体锂金属电解质
锂金属电池与富镍LiNixCoyMn1-x-yO2 (x >;0.7英寸的阴极有望超越目前锂离子电池的能量密度限制。然而,含有游离活性溶剂的传统电解质极易分解,特别是在锂阳极和高压阴极的界面上。在此,我们开发了一种复合准固体电解质(CQSE),利用硫酸化Al2O3 (S-Al2O3)桥接三乙酸纤维素(CTA)来稳定电解质和电极之间的界面。S-Al2O3通过与阴离子的配位相互作用竞争性地解离Li+,促进形成独特的溶剂化结构,其特征是普遍存在的离子对和聚集。此外,S-Al2O3与CTA的配位形成了S-Al2O3桥接的CTA分子链网络,提高了CQSE的机械强度,并固定了自由液体分子。在室温下,CQSE的抗拉强度高达7.4 MPa,离子电导率高达1.8 × 10-3 S cm-1。此外,CQSE不仅可以抑制电极-电解质的副反应,还可以形成富含无机的固体/阴极电解质界面。结果表明,Li|CQSE| lini0.83 co0.11 mn0.060 o2 (NCM83)电池在1℃下循环1000次后仍能保持84%的容量,而袋状电池在0.5℃下循环250次后仍能保持80%的容量。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
文献相关原料
公司名称
产品信息
阿拉丁
lithium bis(trifluoromethanesulfonyl)imide (LiTFSI)
阿拉丁
triethyl phosphate (TEP)
阿拉丁
fluoroethylene carbonate (FEC)
阿拉丁
poly(ethylene oxide) (PEO)
阿拉丁
anhydrous acetonitrile (CH3CN)
阿拉丁
cellulose triacetate
阿拉丁
dichloromethane
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


