Novel quaternary ammonium Gemini surfactant as a highly efficient inhibitor for the corrosion of steel in HCl and H2SO4 solutions
IF 11.2
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
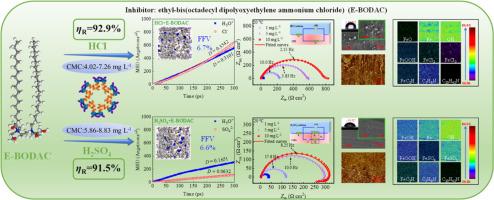
Ethyl-bis(octadecyl dipolyoxyethylene ammonium chloride) (E-BODAC) was a novel quaternary Gemini surfactant and was first found and identified as an excellent inhibitor with excellent surface activity and suitability. The ethoxy group enhances its hydrophilicity, and facilitates a dense adsorption film on metal surface. The inhibitive action and mechanism of E-BODAC on cold rolled steel (CRS) in HCl and H2SO4 solutions was studied in detail by theoretical and experimental methods. The surface activity and degree of polymerization of the surfactant are tightly related to the anti-corrosion properties. HCl has a lower critical micelle concentration (CMC) than H2SO4 solutions. The hydrophilic group in E-BODAC is the preferred site for donating and accepting electrons, with chemical interactions occurring among O, C, and Fe. The addition of E-BODAC obeys Langmuir isotherm, which can drastically reduce the diffusion of H3O+, Cl−/SO42−. E-BODAC has superior corrosion inhibition performance and lower dosage, and the inhibition efficiency for CRS reaches 92.9% in 1.0 M HCl with only 10 mg L−1 of E-BODAC and 91.5% in 0.50 M H2SO4. The inhibition is through physical and chemical adsorption to form a protective film that covers the reaction site, blocking the corrosion reaction. CLSM, SEM, AFM, XPS, and time-of-flight secondary ion mass spectrometer (TOF-SIMS) provide evidence of the adsorption of E-BODAC, efficiently slowing down the corrosion of CRS surface. E-BODAC always shows higher efficiency in HCl than in H2SO4, which is attributed to the re-dissolution of the adsorbed film in the H2SO4 solution. Besides, SO42− ions are larger and migrate more slowly than Cl− ions, resulting in weak adsorption on the metal surface. The assessment of E-BODAC's suitability and durability in different corrosive environments provides the opportunity to determine its optimum conditions and applicability to maximize its corrosion inhibition effect.
新型季铵盐Gemini表面活性剂在HCl和H2SO4溶液中作为钢的高效缓蚀剂
乙基双十八烷基二聚氧乙烯氯化铵(E-BODAC)是一种新型的季型Gemini表面活性剂,首次被发现并被鉴定为具有优良的表面活性和适用性的缓蚀剂。乙氧基增强了其亲水性,有利于在金属表面形成致密的吸附膜。通过理论和实验研究了E-BODAC对冷轧钢(CRS)在HCl和H2SO4溶液中的抑制作用及其机理。表面活性剂的表面活性和聚合度与防腐性能密切相关。HCl溶液的临界胶束浓度(CMC)低于H2SO4溶液。E-BODAC中的亲水性基团是提供和接受电子的首选位点,在O、C和Fe之间发生化学相互作用。E-BODAC的加入符合Langmuir等温线,可以显著降低h30 +, Cl−/SO42−的扩散。E-BODAC具有较好的缓蚀性能和较低的缓蚀用量,在1.0 M HCl条件下,E-BODAC的缓蚀率为92.9%,在0.50 M H2SO4条件下,E-BODAC的缓蚀率为91.5%。缓蚀作用是通过物理和化学吸附形成一层保护膜,覆盖在反应部位,阻断腐蚀反应。CLSM, SEM, AFM, XPS和飞行时间二次离子质谱仪(TOF-SIMS)提供了E-BODAC吸附的证据,有效地减缓了CRS表面的腐蚀。E-BODAC在HCl中的效率始终高于在H2SO4中的效率,这是由于吸附膜在H2SO4溶液中的再溶解。SO42−离子比Cl−离子体积更大,迁移速度更慢,在金属表面的吸附较弱。评价E-BODAC在不同腐蚀环境中的适用性和耐久性,为确定其最佳条件和适用性提供了机会,以最大限度地提高其缓蚀效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Materials Science & Technology
工程技术-材料科学:综合
CiteScore
20.00
自引率
11.00%
发文量
995
审稿时长
13 days
期刊介绍:
Journal of Materials Science & Technology strives to promote global collaboration in the field of materials science and technology. It primarily publishes original research papers, invited review articles, letters, research notes, and summaries of scientific achievements. The journal covers a wide range of materials science and technology topics, including metallic materials, inorganic nonmetallic materials, and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


