High-entropy optimizing d-orbital electronic configuration of metal organic framework for high-current-density anion exchange membrane water electrolysis
IF 16.8
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
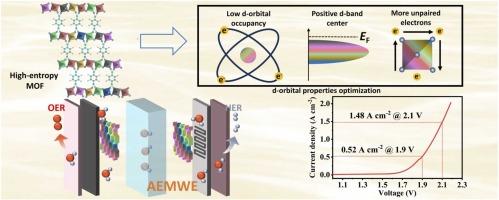
Water electrolysis provides a promising way for hydrogen production through renewable power sources. The exploration of non-precious metal-based electrocatalysts capable of sustaining high current densities for water electrocatalysis is of critical importance. Herein, we develop a high-entropy Mil53 metal organic framework (denoted as Mil53-HE) bifunctional electrocatalyst with improved performance for overall water splitting at large current densities. The improved activity and stability of Mil53-HE for water electrolysis stem from the optimized electronic configurations of d-orbitals in the metal centers, as the overall d-band center (d) is upshifted and the total number of d-orbital electrons in the supercell (∑Nd) is decreased of Mil53-HE. Therefore, the reduced reaction energy barriers and enriched unpaired d-electrons promote both the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER). As a result, the HER and OER activities of Mil53-HE surpass those of their benchmarks Pt/C and RuO2, respectively. Meanwhile, the HER and OER mechanisms on Mil53-HE are revealed by in-situ characterizations and theoretical calculations. Furthermore, the anion exchange membrane water electrolysis cell with Mil53-HE can stably operate at large current densities with small voltages (1.9 V at 0.52 A cm−2 and 2.1 V at 1.48 A cm−2), demonstrating good feasibility for practical application.
高电流密度阴离子交换膜电解金属有机骨架的高熵优化d轨道电子构型
水电解为利用可再生能源制氢提供了一条很有前途的途径。探索能够维持高电流密度的非贵金属电催化剂用于水电催化是至关重要的。在此,我们开发了一种高熵的Mil53金属有机框架(表示为Mil53- he)双功能电催化剂,其在大电流密度下具有更好的整体水分解性能。Mil53-HE的活性和稳定性的提高源于金属中心d轨道电子构型的优化,即Mil53-HE的总d带中心(E′d)上移,超级单体中d轨道电子总数(∑Nd)减少。因此,反应能垒的降低和未配对d电子的富集促进了析氧反应(OER)和析氢反应(HER)。因此,Mil53-HE的HER和OER活性分别超过了其基准Pt/C和RuO2。同时,通过原位表征和理论计算揭示了Mil53-HE的HER和OER机制。此外,含有Mil53-HE的阴离子交换膜电解池可以在大电流密度和小电压(0.52 A cm-2时1.9 V和1.48 A cm-2时2.1 V)下稳定工作,具有良好的实际应用可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


