Spatial microenvironment enhanced photocatalytic reduction of uranyl ions under solar light irradiation
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Photocatalytic reduction of uranyl ions (UO22+) is an environmentally friendly, energy efficient, and highly effective method for uranium-containing wastewater treatment and uranium recovery. Herein, a novel photocatalytic material CH-8 @NNFO-4 with abundant oxygen vacancies was synthesize by growing Ca(OH)2 on the surface of Fe doped NaNbO3 in situ. The Ca(OH)2 synergizes with the oxygen vacancies, creating a microenvironment that narrows the bandgap and extends the light response range. At the same time, the Ca-O enhance carrier transport rates and reduce the electron-hole recombination rate. Under solar irradiation, over 93.68 % UO22+ is rapidly reduced to insoluble U(IV) without protectants or free radical scavengers, which is sixteen times that of pure NaNbO3, with a theoretical reduction capacity reached to 826.45 mg·g⁻1. After five cycles, the removal efficiency remains at 88.77 %. The CH-8 @NNFO-4 possesses excellent recyclability, acid and alkali resistant, anti-interference of anions and cations and chemical stability, Furthermore, the charge transfer in CH-8 @NNFO-4 revealed through DFT calculations, and an enhanced mechanism for the Ca-O synergizes spatial microenvironment photocatalytic reduction of U(VI) was proposed. This work provides a new catalytic reaction design strategy for the efficient reduction of U(VI).
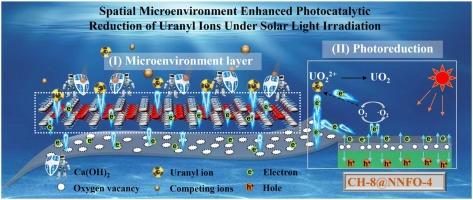
空间微环境增强太阳光照下铀酰离子光催化还原
光催化还原铀酰离子(UO22+)是一种环保、节能、高效的含铀废水处理和铀回收方法。本文通过在Fe掺杂的NaNbO3表面原位生长Ca(OH)2,合成了一种具有丰富氧空位的新型光催化材料CH-8@NNFO-4。Ca(OH)2与氧空位协同作用,形成一个微环境,缩小了带隙,扩大了光响应范围。同时,Ca-O提高了载流子输运速率,降低了电子-空穴复合速率。在太阳照射下,超过93.68%的UO22+在没有保护剂和自由基清除剂的情况下被迅速还原为不溶性的U(IV),是纯NaNbO3的16倍,理论还原能力达到826.45 mg·g毒血症。循环5次后,去除率保持在88.77%。CH-8@NNFO-4具有优异的可回收性、耐酸碱性、抗阴离子和阳离子干扰性和化学稳定性。此外,通过DFT计算揭示了CH-8@NNFO-4中的电荷转移,并提出了Ca-O协同空间微环境光催化还原U(VI)的增强机制。本研究为高效还原U(VI)提供了一种新的催化反应设计策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


