A guanidinium-based ionic COF for selective and efficient capture of 99TcO4−: A synergistic effect of ion exchange and hydrogen bond
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
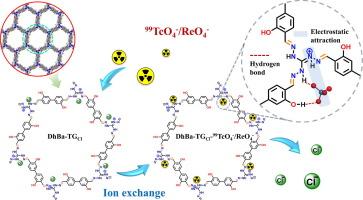
The efficient capture of 99Tc from radioactive wastewater is crucial for the sustainable development of nuclear energy and human safety. In this study, a guanidinium-based ionic covalent organic framework (DhBa-TGCl) was synthesized using 1,2,3-Triaminoguanidine hydrochloride (TGCl) and 3,3′-dihydroxy-[1,1-biphenyl]-4,4′-dicarboxaldehyde (DhBa) to capture 99TcO4−/ReO4− in aqueous solutions. Batch experiments were conducted to investigate the effects of solution pH, adsorbent concentration, contact time, initial concentration and competing anions. The experiments demonstrated that within the pH range of 3–10, the removal efficiency of ReO4−, an analogue of 99TcO4−, remained stable, with rapid adsorption kinetics (over 80 % of 99TcO4− removed within 30 s) and a high adsorption capacity (227.21 mg⋅g−1 for ReO4−). Furthermore, competition experiments in binary systems with excess coexisting anions (Cl−, NO3−, SO42−, and MoO42−) and the DFT calculation results revealed that DhBa-TGCl maintained good selectivity for ReO4− and 99TcO4−. Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) analyses indicated that the adsorption mechanism involved a synergistic effect of electrostatic attraction and hydrogen bonding. Additionally, DhBa-TGCl can withstand 200 kGy of γ-ray irradiation and demonstrates excellent reusability after five adsorption–desorption cycles. The excellent properties of DhBa-TGCl highlight its great potential for rapid and selective removal of 99TcO4− from radioactive waste liquids.

一种选择性高效捕获99TcO4−的胍基离子COF:离子交换和氢键的协同效应
从放射性废水中高效捕获99Tc对核能的可持续发展和人类安全至关重要。在本研究中,以1,2,3-三氨基胍盐酸盐(TGCl)和3,3 ' -二羟基-[1,1-联苯]-4,4 ' -二甲醛(DhBa)为原料合成了胍基离子共价有机骨架(DhBa-TGCl),用于在水溶液中捕获99TcO4−/ReO4−。通过批量实验考察了溶液pH、吸附剂浓度、接触时间、初始浓度和竞争阴离子对吸附效果的影响。实验表明,在3 ~ 10的pH范围内,ReO4−(99TcO4−的类似物)的去除效率保持稳定,吸附动力学快速(30 s内99TcO4−的去除率超过80% %),吸附量高(227.21 mg•g−1)。此外,在过量阴离子(Cl−、NO3−、SO42−和MoO42−)共存的二元体系中进行的竞争实验和DFT计算结果表明,DhBa-TGCl对ReO4−和99TcO4−保持良好的选择性。傅里叶变换红外光谱(FTIR)和x射线光电子能谱(XPS)分析表明,吸附机理涉及静电吸引和氢键的协同作用。此外,DhBa-TGCl可以承受200 kGy的γ射线照射,并在5次吸附-解吸循环后表现出良好的可重复使用性。DhBa-TGCl的优异性能突出了它在快速和选择性去除放射性废液中的99TcO4−方面的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


