Modulation of inter-elemental synergy and oxygen vacancy content of CdZrOx solid solution catalysts by Ga for effective CO2 hydrogenation to methanol
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
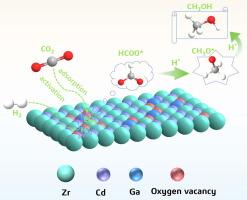
CO2 conversion into valuable chemical products such as methanol using green hydrogen generated from renewable energy sources is an effective way to reduce CO2 emissions. However, preparing catalysts with high activity and methanol yield remains a significant challenge. Here, we found that the synergistic effect of Ga, Cd and Zr produced by introducing Ga into CdZrOx solid solution catalysts significantly facilitated the methanol synthesis from CO2 hydrogenation. The Ga-promoted CdZrOx solid solution catalyst (5 %GaCdZrOx) exhibited a CO2 conversion of 8.6 % and a methanol space time yield of 457 mg/gcat·h, which is remarkably higher than the unmodified CdZrOx, and displayed excellent long-term stability. Multiple characterization results indicate that Ga acts as a promoter, leading to changes in the electronic structure of the CdZrOx solid solution, which generates a large number of oxygen vacancies on the catalyst surface, and thus promotes the methanol generation. A series of chemisorption experiments and in situ DRIFTS revealed that the Ga, Cd and Zr components in the 5 %GaCdZrOx solid solution catalyst exhibited a stronger synergistic effect with enhanced CO2 and H2 adsorption and activation compared to CdZrOx. Both the strengthened synergistic effect of the solid solution structure and the elevated oxygen vacancies promoted the adsorption of CO2 and the formation of more CO3* species, while increased H2 adsorption and activation capacity further accelerated the hydrogenation of CO3* species into HCOO* and CH3O* species. This study provides specific insights into the modification of solid solution catalysts with bimetallic oxide components for the CO2 hydrogenation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


