Anti-inflammatory 5,6,7,8-tetrahydro-2-(2-phenylethyl) chromone derivatives from the stems of Aquilaria sinensis
IF 2.5
3区 医学
Q3 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Three previously undescribed 5,6,7,8-tetrahydro-2-(2-phenylethyl)chromones, (5S,6R,7S,8S)-8-chloro-5-ethoxy-6,7-dihydroxy-2-[2-(4′-methoxyphenyl)ethyl]-5,6,7,8-tetrahydrochromone (1), (5R,6S,7R,8R)-8-chloro-5-ethoxy-6,7-dihydroxy-2-(2- phenylethyl)-5,6,7,8-tetrahydrochromone (2), (5S,6R,7S,8R)-5,8-dichloro-6,7- dihydroxy-2-[2-(4′-methoxyphenyl)ethyl]-5,6,7,8-tetrahydrochromone (3), and 28 known analogues (4–31) were isolated from the stems of Aquilaria sinensis. Their structures were characterized by the spectroscopic methods, and the absolute configuration was resolved by circular dichroism (CD) spectroscopy. Bioactivity evaluation indicated that compounds 3–8 had significant inhibition effect in the production of NO in an inflammatory cell model with relatively lower IC50 values of 5.54, 11.44, 3.68, 7.15, 10.26 and 13.04 μM, respectively, compared to the positive control indomethacin (IC50 = 23.03 μM).
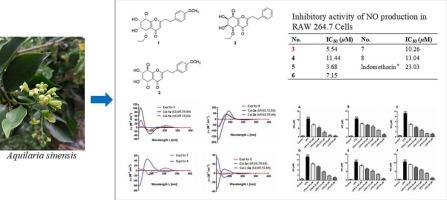
从旱莲草茎中提取的 5,6,7,8-四氢-2-(2-苯基乙基)铬酮衍生物具有抗炎作用。
(5R,6S,7R,8R)-8-氯-5-乙氧基-6,7-二羟基-2-(2-苯基乙基)-5,6,7,8-四氢苯并吡喃酮(2)、(5S,6R,7S,8R)-5,8-二氯-6,7-二羟基-2-[2-(4'-甲氧基苯基)乙基]-5,6,7,8-四氢苯并吡喃酮(3),以及 28 种已知的类似物(4-31)。通过光谱方法对它们的结构进行了表征,并通过圆二色性(CD)光谱解析了它们的绝对构型。生物活性评价表明,化合物 3-8 在炎症细胞模型中对 NO 的产生有显著的抑制作用,与阳性对照吲哚美辛(IC50 = 23.03 μM)相比,其 IC50 值分别为 5.54、11.44、3.68、7.15、10.26 和 13.04 μM,相对较低。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fitoterapia
医学-药学
CiteScore
5.80
自引率
2.90%
发文量
198
审稿时长
1.5 months
期刊介绍:
Fitoterapia is a Journal dedicated to medicinal plants and to bioactive natural products of plant origin. It publishes original contributions in seven major areas:
1. Characterization of active ingredients of medicinal plants
2. Development of standardization method for bioactive plant extracts and natural products
3. Identification of bioactivity in plant extracts
4. Identification of targets and mechanism of activity of plant extracts
5. Production and genomic characterization of medicinal plants biomass
6. Chemistry and biochemistry of bioactive natural products of plant origin
7. Critical reviews of the historical, clinical and legal status of medicinal plants, and accounts on topical issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


