Comparative validation of UV-spectrophotometry and RP-HPLC methods for cefixime and moxifloxacin analysis
IF 2.6
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Aim
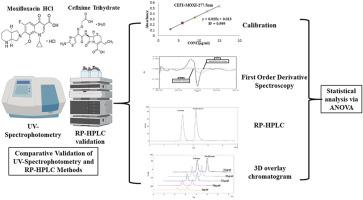
This study presents the development and validation of UV-spectrophotometry and RP-HPLC methods for the simultaneous quantification of Cefixime Trihydrate (CEFI) and Moxifloxacin Hydrochloride (MOXI) in pharmaceutical formulations.
Methodology
Two UV-spectrophotometric methods, including the absorbance ratio (Q-Absorption) and First Order Derivative Spectroscopy, were developed and validated for their linearity, precision, accuracy, and sensitivity. Additionally, a robust RP-HPLC method using a C18 column and optimized mobile phase was employed for efficient separation and simultaneous estimation of CEFI and MOXI. All methods were validated in accordance with ICH guidelines, with system suitability parameters confirming the reliability of the RP-HPLC method for routine analysis.
Results
The absorbance ratio and First Order Derivative methods demonstrated low %R.S.D values, high accuracy, and satisfactory sensitivity for both drugs. Similarly, the RP-HPLC method achieved high resolution, precision, and robustness. Statistical analysis through ANOVA revealed no significant differences between the methods in terms of accuracy and precision. The methods were applied to analyze marketed formulations, further confirming their applicability in routine quality control.
Conclusion
In conclusion, the validated methods provide accurate, precise, and sensitive techniques for the simultaneous estimation of CEFI and MOXI, making them suitable for pharmaceutical quality control and regulatory compliance.
紫外分光光度法和 RP-HPLC 法用于头孢克肟和莫西沙星分析的比较验证
目的:本研究建立并验证了紫外分光光度法和 RP-HPLC 法同时定量检测药物制剂中三水头孢克肟(CEFI)和盐酸莫西沙星(MOXI)的方法:开发了两种紫外分光光度法,包括吸光度比(Q-吸收)和一阶衍射光谱法,并对其线性、精密度、准确度和灵敏度进行了验证。此外,还采用了一种稳健的 RP-HPLC 方法,使用 C18 色谱柱和优化的流动相,对 CEFI 和 MOXI 进行高效分离和同时估测。所有方法均按照 ICH 指南进行了验证,系统适用性参数证实了 RP-HPLC 方法在常规分析中的可靠性:结果:吸光度比值法和一阶衍射法对这两种药物都显示出较低的%R.S.D 值、较高的准确度和令人满意的灵敏度。同样,RP-HPLC 方法也实现了高分辨率、高精度和高稳定性。通过方差分析进行统计分析后发现,两种方法在准确度和精密度方面没有显著差异。这些方法被用于分析市场上的制剂,进一步证实了它们在常规质量控制中的适用性:总之,经过验证的方法为同时估算 CEFI 和 MOXI 提供了准确、精确和灵敏的技术,使其适用于药品质量控制和法规遵从。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytical biochemistry
生物-分析化学
CiteScore
5.70
自引率
0.00%
发文量
283
审稿时长
44 days
期刊介绍:
The journal''s title Analytical Biochemistry: Methods in the Biological Sciences declares its broad scope: methods for the basic biological sciences that include biochemistry, molecular genetics, cell biology, proteomics, immunology, bioinformatics and wherever the frontiers of research take the field.
The emphasis is on methods from the strictly analytical to the more preparative that would include novel approaches to protein purification as well as improvements in cell and organ culture. The actual techniques are equally inclusive ranging from aptamers to zymology.
The journal has been particularly active in:
-Analytical techniques for biological molecules-
Aptamer selection and utilization-
Biosensors-
Chromatography-
Cloning, sequencing and mutagenesis-
Electrochemical methods-
Electrophoresis-
Enzyme characterization methods-
Immunological approaches-
Mass spectrometry of proteins and nucleic acids-
Metabolomics-
Nano level techniques-
Optical spectroscopy in all its forms.
The journal is reluctant to include most drug and strictly clinical studies as there are more suitable publication platforms for these types of papers.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


