Bilateral transcranial direct-current stimulation confers neuroprotection through suppression of PKM2 after mouse cerebral ischemia injury
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Background
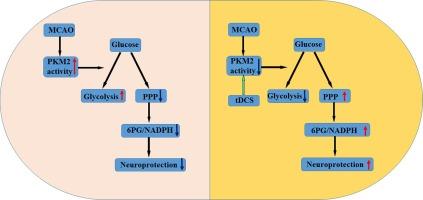
In its tetrameric form, pyruvate kinase M2 isoform (PKM2) catalyzes the last step of glycolysis and plays a key role in the metabolic reprogramming via regulating the signaling of pentose phosphate pathway (PPP). But the role of PKM2 in cerebral ischemia–reperfusion (I/R) injury remains unknown.
Methods
Mice model of middle cerebral artery occlusion (MCAO) and model of oxygen-glucose deprivation (OGD) injury in cultured neurons were established. PKM2 activator or inhibitor were used to test the effects of PKM2 in wild-type and PKM2 (−/-) mice after I/R injury. Biochemical and molecular approach were used to detect the level of PKM2 tetramers and PPP metabolites.
Results
We showed for the first time that ischemia-induced increase of PKM2 activity promoted neuronal death via the suppression of PPP-dependent antioxidant capacity. To identify therapeutic approach that suppresses ischemia-induced increase of PKM2 activity, we tested the effect of bilateral transcranial direct-current stimulation (BtDCS), a newly established BtDCS approach by us, on PKM2 activity after mouse I/R. Our data demonstrated that BtDCS inhibited PKM2 activity in the ischemic neurons. BtDCS also reduced the cerebral infarct volume and the neurological deficits in stroke mice. We found that BtDCS-induced neuroprotection was mediated through the suppression of PKM2 activity after I/R.
Conclusions
Together, this study provided novel evidence that supported PKM2 as a crucial regulator of neuronal metabolism after cerebral I/R injury, and revealed the molecular mechanism by which BtDCS protects against mouse cerebral I/R injury through regulating PKM2-mediated metabolic reprogramming.
双侧经颅直流电刺激通过抑制小鼠脑缺血损伤后的 PKM2 发挥神经保护作用
背景:丙酮酸激酶M2异构体(PKM2)以四聚体形式催化糖酵解的最后一步,并通过调节磷酸戊糖途径(PPP)的信号传导在代谢重编程中发挥关键作用。但PKM2在脑缺血再灌注(I/R)损伤中的作用仍然未知:方法:建立大脑中动脉闭塞(MCAO)小鼠模型和氧-葡萄糖剥夺(OGD)损伤培养神经元模型。用PKM2激活剂或抑制剂检测野生型和PKM2(-/-)小鼠I/R损伤后PKM2的作用。采用生化和分子方法检测PKM2四聚体和PPP代谢物的水平:结果:我们首次发现缺血诱导的 PKM2 活性增加会通过抑制 PPP 依赖性抗氧化能力促进神经元死亡。为了找到抑制缺血诱导的PKM2活性增加的治疗方法,我们测试了双侧经颅直流电刺激(BtDCS)对小鼠I/R后PKM2活性的影响。我们的数据表明,BtDCS抑制了缺血神经元中PKM2的活性。BtDCS 还能缩小脑梗塞体积,减轻中风小鼠的神经功能缺损。我们发现,BtDCS诱导的神经保护作用是通过抑制I/R后的PKM2活性介导的:总之,这项研究提供了新的证据,支持PKM2是脑I/R损伤后神经元代谢的关键调节因子,并揭示了BtDCS通过调节PKM2介导的代谢重编程来保护小鼠脑I/R损伤的分子机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


