Electrocyclization for the Synthesis of Mono- and Disulfonyl-substituted Pyrazoles From Sulfonyl Hydrazines and 1,3-Diketones
IF 1.9
4区 化学
Q3 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The tunable electrocyclic reactions for the synthesis of a series of mono- and disulfonyl-substituted pyrazoles from sulfonyl hydrazines and 1,3-diketones were investigated by utilizing inexpensive electrolytes under electrochemical conditions in an undivided cell. When nBu4NBF4 was used as an electrolyte, it afforded monosulfonyl-substituted pyrazoles. By contrast, when NH4I was used as an electrolyte, it gave disulfonyl-substituted pyrazoles. The mechanistic investigation shows that they all underwent a free radical process but formed N-centered sulfonyl hydrazine radical and S-centered sulfonyl radical in the synthesis of mono- and disulfonyl-substituted pyrazoles, respectively.
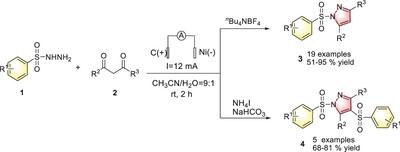
从磺酰基肼和 1,3-二酮电环化合成单磺酰基和二磺酰基取代的吡唑
研究人员利用廉价的电解质,在不分流电池的电化学条件下,研究了从磺酰基肼和 1,3 二甲基酮合成一系列单磺酰基和二磺酰基取代吡唑的可调电环反应。当使用 nBu4NBF4 作为电解质时,可以得到单磺酰基取代的吡唑。相反,当使用 NH4I 作为电解质时,则会产生二磺酰基取代的吡唑。机理研究表明,在合成单磺酰基和二磺酰基取代的吡唑时,它们都经历了一个自由基过程,但分别形成了 N-中心磺酰肼自由基和 S-中心磺酰基自由基。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ChemistrySelect
Chemistry-General Chemistry
CiteScore
3.30
自引率
4.80%
发文量
1809
审稿时长
1.6 months
期刊介绍:
ChemistrySelect is the latest journal from ChemPubSoc Europe and Wiley-VCH. It offers researchers a quality society-owned journal in which to publish their work in all areas of chemistry. Manuscripts are evaluated by active researchers to ensure they add meaningfully to the scientific literature, and those accepted are processed quickly to ensure rapid online publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


