MOFs derived Ni-Mn bimetal nano-catalysts with enhanced hydrogen pump effect for boosting hydrogen sorption performance of MgH2
IF 15.8
1区 材料科学
Q1 METALLURGY & METALLURGICAL ENGINEERING
引用次数: 0
Abstract
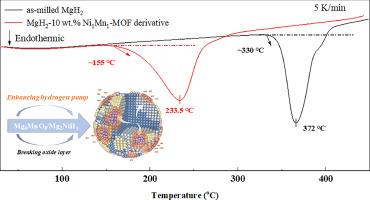
In the present work, highly effective Ni-MnO binary nanocomposite catalysts were designed and synthesized using a one-pot method from Ni-Mn based bi-metal organic frameworks (MOFs). These nanocomposites were introduced into MgH2 through ball milling as catalysts to enhance the hydrogen storage properties of MgH2. Through varying the Ni/Mn ratio in the bimetal MOFs, it is found that the Ni1Mn1−MOF derived catalyst showed the best promotion effect on MgH2. The MgH2–10 wt.% Ni1Mn1−MOF derivative demonstrated favorable overall performance with the low desorption peak temperature (218.2 °C) with a saturated hydrogen capacity of 6.42 wt.% and rapid hydrogen release/uptake kinetics. It can still reabsorb about 1.15 wt.% H2 within 30 min at a temperature as low as 50 °C. Both performance tests (DSC and TPD) and structural characterizations (XRD, HRTEM, etc.) revealed that the synergistic role of in situ formed Mg6MnO8 and Mg2NiH4/Mg2Ni phases for improving the hydrogen sorption properties of MgH2. Theoretical calculations reveal that Mg6MnO8 destabilizes metal-H bonds in MgH2 and Mg2NiH4, leading to an enhanced “hydrogen pump” effect of Mg2NiH4 for MgH2. This research provides a strategy to rational design and preparation of bimetal MOF derivatives for the development of advanced hydrogen storage materials.
具有增强氢泵效应的 MOFs 衍生镍锰双金属纳米催化剂可提高 MgH2 的吸氢性能
在本研究中,利用镍-锰双金属有机框架(MOFs),采用一锅法设计并合成了高效的镍-锰氧化物二元纳米复合催化剂。通过球磨将这些纳米复合材料引入 MgH2 作为催化剂,以提高 MgH2 的储氢性能。通过改变双金属 MOF 中的镍/锰比例,发现镍1锰1-MOF 衍生催化剂对 MgH2 的促进效果最好。MgH2-10 wt.% Ni1Mn1-MOF 衍生物的整体性能良好,解吸峰温度低(218.2 °C),饱和氢容量为 6.42 wt.%,氢气释放/吸收动力学迅速。在温度低至 50 °C 的情况下,它仍能在 30 分钟内重新吸收约 1.15 重量百分比的 H2。性能测试(DSC 和 TPD)和结构表征(XRD、HRTEM 等)均表明,原位形成的 Mg6MnO8 和 Mg2NiH4/Mg2Ni 相对改善 MgH2 的吸氢性能具有协同作用。理论计算显示,Mg6MnO8 会破坏 MgH2 和 Mg2NiH4 中金属-H 键的稳定性,从而增强 Mg2NiH4 对 MgH2 的 "氢泵 "效应。这项研究为合理设计和制备双金属 MOF 衍生物以开发先进的储氢材料提供了一种策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Magnesium and Alloys
Engineering-Mechanics of Materials
CiteScore
20.20
自引率
14.80%
发文量
52
审稿时长
59 days
期刊介绍:
The Journal of Magnesium and Alloys serves as a global platform for both theoretical and experimental studies in magnesium science and engineering. It welcomes submissions investigating various scientific and engineering factors impacting the metallurgy, processing, microstructure, properties, and applications of magnesium and alloys. The journal covers all aspects of magnesium and alloy research, including raw materials, alloy casting, extrusion and deformation, corrosion and surface treatment, joining and machining, simulation and modeling, microstructure evolution and mechanical properties, new alloy development, magnesium-based composites, bio-materials and energy materials, applications, and recycling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


