Fungal symbiont transmitted by free-living mice promotes type 2 immunity
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
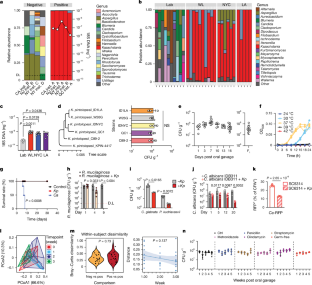
The gut mycobiota is crucial for intestinal homeostasis and immune function1. Yet its variability and inconsistent fungal colonization of laboratory mice hinders the study of the evolutionary and immune processes that underpin commensalism2,3. Here, we show that Kazachstania pintolopesii is a fungal commensal in wild urban and rural mice, with an exceptional ability to colonize the mouse gastrointestinal tract and dominate the gut mycobiome. Kazachstania pintolopesii colonization occurs in a bacteria-independent manner, results in enhanced colonization resistance to other fungi and is shielded from host immune surveillance, allowing commensal presence. Following changes in the mucosal environment, K. pintolopesii colonization triggers a type 2 immune response in mice and induces gastrointestinal eosinophilia. Mechanistically, we determined that K. pintolopesii activates type 2 immunity via the induction of epithelial IL-33 and downstream IL-33–ST2 signalling during mucus fluctuations. Kazachstania pintolopesii-induced type 2 immunity enhanced resistance to helminth infections or aggravated gastrointestinal allergy in a context-dependent manner. Our findings indicate that K. pintolopesii is a mouse commensal and serves as a valuable model organism for studying gut fungal commensalism and immunity in its native host. Its unnoticed presence in mouse facilities highlights the need to evaluate its influence on experimental outcomes and phenotypes. Kazachstania pintolopesii is a highly prevalent fungal symbiont that can trigger type 2 immunity and influence the composition of the gut mycobiome, as well as immune and disease phenotypes.

自由生活的小鼠传播的真菌共生体可促进 2 型免疫力
肠道真菌生物群对肠道平衡和免疫功能至关重要1。然而,实验室小鼠真菌定植的多变性和不一致性阻碍了对共生的进化和免疫过程的研究2,3。在这里,我们发现Kazachstania pintolopesii是城市和农村野生小鼠的共生真菌,它具有在小鼠胃肠道定殖并主导肠道真菌生物群的特殊能力。Kazachstania pintolopesii的定殖不依赖于细菌,能增强对其他真菌的定殖抵抗力,并且不受宿主免疫监视,从而使共生菌得以存在。随着粘膜环境的变化,K. pintolopesii的定植会引发小鼠的2型免疫反应,并诱发胃肠道嗜酸性粒细胞增多。从机理上讲,我们确定K. pintolopesii通过诱导上皮细胞IL-33和粘液波动过程中的下游IL-33-ST2信号激活2型免疫。Kazachstania-pintolopesi诱导的2型免疫增强了对蠕虫感染的抵抗力,或以环境依赖的方式加重了胃肠道过敏。我们的研究结果表明,K. pintolopesii是一种小鼠共生真菌,是研究其原生宿主肠道真菌共生和免疫的重要模式生物。它在小鼠设施中的存在并未引起人们的注意,这突出表明有必要评估它对实验结果和表型的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


