Antitumour vaccination via the targeted proteolysis of antigens isolated from tumour lysates
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
The activation of cytotoxic T cells against tumour cells typically requires the cross-presentation, by antigen-presenting cells (and via major histocompatibility complex class I molecules), of an epitope derived from a tumour antigen. A critical step in antigen processing is the proteolysis of tumour antigens mediated by the ubiquitin–proteasome pathway. Here we describe a tumour vaccine leveraging targeted antigen degradation to augment antigen processing and cross-presentation. Analogous to proteolysis-targeting chimaeras, the vaccine consists of lymph-node-targeting lipid nanoparticles encapsulated with tumour antigens pre-conjugated with ligands that can bind to E3 ubiquitin ligases. In mice with subcutaneous human melanoma or triple-negative breast cancer, or with orthotopic mouse Lewis lung carcinoma or clinically inoperable mouse ovarian cancer, subcutaneously delivered vaccines prepared using tumour lysate proteins elicited antigen-specific adaptive immunity and immunological memory, and inhibited tumour growth, metastasis and recurrence, particularly when combined with immune checkpoint inhibition. A tumour vaccine leveraging the targeted degradation of antigens isolated from tumour lysates augmented antigen processing and cross-presentation, and inhibited tumour growth, metastasis and recurrence in multiple mouse models of cancers.

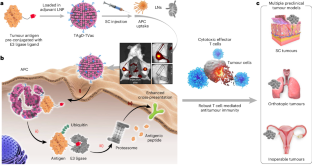
通过对从肿瘤裂解物中分离出来的抗原进行靶向蛋白水解来接种抗肿瘤疫苗
激活细胞毒性 T 细胞对抗肿瘤细胞通常需要抗原递呈细胞(通过主要组织相容性复合体 I 类分子)交叉递呈来自肿瘤抗原的表位。抗原处理的一个关键步骤是通过泛素蛋白酶体途径对肿瘤抗原进行蛋白水解。在这里,我们描述了一种利用靶向抗原降解来增强抗原处理和交叉呈递的肿瘤疫苗。与蛋白酶靶向chimaeras类似,该疫苗由淋巴结靶向脂质纳米颗粒组成,其中封装有肿瘤抗原,并预先与能与E3泛素连接酶结合的配体结合。在患有皮下人类黑色素瘤或三阴性乳腺癌的小鼠,或患有正位小鼠刘易斯肺癌或临床上无法手术的小鼠卵巢癌的小鼠中,使用肿瘤裂解物蛋白制备的皮下注射疫苗激发了抗原特异性适应性免疫和免疫记忆,并抑制了肿瘤的生长、转移和复发,尤其是在与免疫检查点抑制相结合时。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
文献相关原料
公司名称
产品信息
索莱宝
Bicinchoninic acid disodium salt hydrate (BCA)
索莱宝
Bicinchoninic acid disodium salt hydrate
索莱宝
Bicinchoninic acid disodium salt hydrate (BCA)
Sigma
FITC
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


