Strategic Design for High-Efficiency Oxygen Evolution Reaction (OER) Catalysts by Triggering Lattice Oxygen Oxidation in Cobalt Spinel Oxides
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
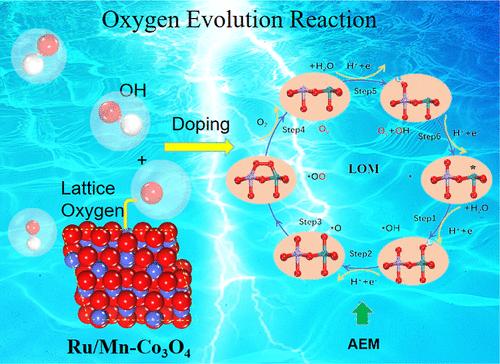
High-efficiency catalysts with refined electronic structures are highly desirable for promoting the kinetics of the oxygen evolution reaction (OER) and enhancing catalyst durability. This study comprehensively explores strategies involving metal doping and oxygen vacancies for enhancing the acidic OER catalytic activity of Co3O4. Through extensive screening of 3d and 4d transition metals using density functional theory (DFT) simulations, we demonstrate that the incorporation of metal dopants and oxygen vacancies into Co3O4 potentially triggers a transition from the adsorbate evolution mechanism (AEM) to the lattice oxygen oxidation mechanism (LOM) in the oxygen evolution reaction (OER). While the formation of the O–O bond in the intermediate *OOH poses challenges, a significantly reduced overpotential facilitates efficient conversion of O to O2 through the LOM in *OH and lattice oxygen. Additionally, we find that Mn doping can significantly improve the stability of the catalyst. Building upon the rationale above, we employed a dual doping strategy in subsequent experiments to enhance both the activity and stability. Our final design involved the codoping of Mn and Ru in Co3O4, along with an appropriate amount of oxygen vacancies. This catalyst demonstrates a low overpotential (η10 = 230 mV) compared to pure Co3O4 and maintains stable operation for over 120 h, representing a 12-fold increase. By exploring and harnessing the LOM, more efficient, stable, and cost-effective OER catalysts can be designed, providing crucial support for technologies such as water electrolysis in clean energy.
通过触发钴尖晶石氧化物中的晶格氧氧化实现高效氧气进化反应 (OER) 催化剂的战略设计
具有精细电子结构的高效催化剂对于促进氧进化反应(OER)的动力学和提高催化剂的耐久性非常重要。本研究全面探讨了提高 Co3O4 酸性 OER 催化活性的金属掺杂和氧空位策略。通过使用密度泛函理论(DFT)模拟对 3d 和 4d 过渡金属进行广泛筛选,我们证明在 Co3O4 中掺入金属掺杂物和氧空位可能会引发氧进化反应(OER)从吸附剂进化机制(AEM)向晶格氧氧化机制(LOM)的转变。虽然中间产物 *OOH 中 O-O 键的形成带来了挑战,但显著降低的过电位有助于通过 *OH 和晶格氧中的 LOM 将 O 有效地转化为 O2。此外,我们还发现掺杂锰可显著提高催化剂的稳定性。基于上述原理,我们在随后的实验中采用了双重掺杂策略,以提高催化剂的活性和稳定性。我们的最终设计包括在 Co3O4 中掺入 Mn 和 Ru 以及适量的氧空位。与纯 Co3O4 相比,这种催化剂具有较低的过电位(η10 = 230 mV),并能稳定运行 120 小时以上,相当于提高了 12 倍。通过探索和利用 LOM,可以设计出更高效、更稳定、更具成本效益的 OER 催化剂,为清洁能源中的电解水等技术提供重要支持。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


