Efficient genome editing using CRISPR/Cas9 technology and its application for identifying Sesquiterpene synthases involved in the biosynthesis of Steperoxides in Steccherinum ochraceum
IF 2.4
3区 生物学
Q3 GENETICS & HEREDITY
引用次数: 0
Abstract
CRISPR technology has been widely used for gene editing in various species,but the genetic manipulation in basidiomycete mushrooms is still notoriously difficult for unknown endogenous promoters and inefficient DNA delivery. Steccherinum ochraceum is a white rot basidiomycete fungus with abundant secondary metabolites and plays an important ecological role worldwide. To facilitate the study of gene function in S. ochraceum, an effective CRISPR/Cas9 system was successfully developed by identifying highly efficient endogenous promoters, and utilizing the Agrobacterium-transformation method. Two efficient endogenous RNA polymerase II promoters (Psogpd and Psotef1) and one efficient RNA polymerase III promoter (Pu6-d) were identified and characterized, with an editing efficiency of 61.5 % at the ura3 locus. Using this optimized system, the sesquiterpene gene A0064, which could produce 10 possible sesquiterpenes in the heterologous expression system of A. oryzae, was knocked out to obtain A0064 knockout strain S. ochraceum (∆A0064). Steperoxide A could not be detected in S. ochraceum (∆A0064), demonstrating that A0064 was the only enzyme responsible for the biosynthesis of β-chamigrene (the sesquiterpene skeleton of steperoxide A) in S. ochraceum. This efficient system will enable precise targeting and multiplex editing of S. ochraceum genes, facilitating functional studies of genes involved in lignin degradation and natural product biosynthesis in S. ochraceum, and providing some valuable guidance for gene editing in tens of thousands of macrofungi.
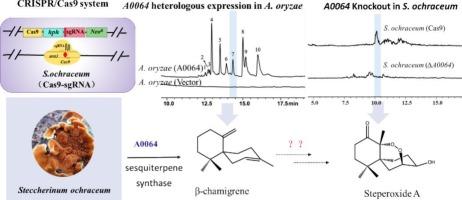
利用 CRISPR/Cas9 技术进行高效基因组编辑并将其应用于鉴定参与 Steccherinum ochraceum 中 Steperoxides 生物合成的倍半萜合成酶。
CRISPR技术已被广泛应用于各种物种的基因编辑,但由于内源启动子未知和DNA传递效率低下,基枝菌的基因操作仍是众所周知的难题。Steccherinum ochraceum 是一种白腐基生真菌,具有丰富的次生代谢产物,在世界范围内发挥着重要的生态作用。为了便于研究赭色链格孢的基因功能,研究人员通过识别高效的内源启动子,并利用农杆菌转化法,成功开发了一套有效的 CRISPR/Cas9 系统。该系统识别并鉴定了两个高效的内源 RNA 聚合酶 II 启动子(Psogpd 和 Psotef1)和一个高效的 RNA 聚合酶 III 启动子(Pu6-d),在 ura3 基因座上的编辑效率为 61.5%。利用这一优化系统,敲除了在 A. oryzae 异源表达系统中可产生 10 种倍半萜的倍半萜基因 A0064,得到了 A0064 基因敲除株 S. ochraceum(ΔA0064)。在 S. ochraceum(ΔA0064)中检测不到菊酯氧化物 A,这表明 A0064 是 S. ochraceum 中负责β-chamigrene(菊酯氧化物 A 的倍半萜骨架)生物合成的唯一酶。这一高效系统将实现对赭色真菌基因的精确靶向和多重编辑,有助于对赭色真菌中参与木质素降解和天然产物生物合成的基因进行功能研究,并为数以万计的大型真菌的基因编辑提供一些有价值的指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fungal Genetics and Biology
生物-遗传学
CiteScore
6.20
自引率
3.30%
发文量
66
审稿时长
85 days
期刊介绍:
Fungal Genetics and Biology, formerly known as Experimental Mycology, publishes experimental investigations of fungi and their traditional allies that relate structure and function to growth, reproduction, morphogenesis, and differentiation. This journal especially welcomes studies of gene organization and expression and of developmental processes at the cellular, subcellular, and molecular levels. The journal also includes suitable experimental inquiries into fungal cytology, biochemistry, physiology, genetics, and phylogeny.
Fungal Genetics and Biology publishes basic research conducted by mycologists, cell biologists, biochemists, geneticists, and molecular biologists.
Research Areas include:
• Biochemistry
• Cytology
• Developmental biology
• Evolutionary biology
• Genetics
• Molecular biology
• Phylogeny
• Physiology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


