Rabies virus-mimicking liposomes for targeted gene therapy in Alzheimer’s disease
IF 5.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
RNA interference (RNAi) harbors significant potential for treating neurological disorders; nevertheless, limited efficacy has been discerned. The presence of barriers within the central nervous system, coupled with the inherent instability of nucleic acids within biological conditions, poses formidable challenges in advancing effective gene delivery strategies. In this study, we designed and prepared a virus-mimic non-viral gene vector, rabies virus glycoprotein (RVG29)-decorated liposome (f(Lipo)-RVG29), to deliver small interfering RNAs to the brain. Alzheimer’s disease (AD) was chosen as a model of neurodegenerative disease in this context, and b-site APP cleaving enzyme silencing siRNA (siBACE1) was used. The developed liposomal delivery system has a particle size of under 80 nm with a spherical shape, positive zeta potential, and the ability to protect siRNA against nucleases. In vitro studies demonstrate that functionalizing the cationic liposome by the RVG29 targeting ligand significantly enhances the effectiveness of gene delivery and silencing. Examination through ex vivo imaging illustrates an increased deposition of fluorescent-labeled f(Lipo)-RVG29 within brain tissue after 12 h post application. Additionally, the in vivo delivery of f(Lipo)-RVG29 carrying siRNA has substantially suppressed BACE1 expression at both mRNA and protein levels within the brain tissue. Our results suggest that the developed non-viral vector could be a promising gene carrier system combining the synergistic effect of virus-mimic RVG29 ligand with bioinspired liposome that imitates the natural lipid bilayers of cell membranes for brain-targeted RNAi therapeutics.
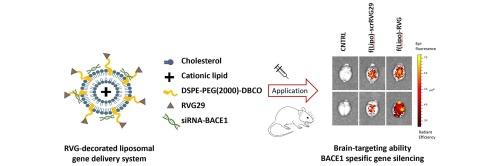
模仿狂犬病毒的脂质体用于阿尔茨海默病的靶向基因治疗。
RNA 干扰(RNAi)在治疗神经系统疾病方面潜力巨大,但疗效有限。中枢神经系统内存在障碍,再加上核酸在生物条件下固有的不稳定性,为推进有效的基因递送策略带来了巨大挑战。在这项研究中,我们设计并制备了一种模拟病毒的非病毒基因载体--狂犬病毒糖蛋白(RVG29)装饰脂质体(f(Lipo)-RVG29),用于向大脑递送小干扰 RNA。本文选择阿尔茨海默病(AD)作为神经退行性疾病的模型,并使用了b位点APP裂解酶沉默siRNA(siBACE1)。所开发的脂质体递送系统粒径小于80纳米,呈球形,zeta电位为正,能够保护siRNA免受核酸酶的侵蚀。体外研究表明,用 RVG29 靶向配体对阳离子脂质体进行功能化处理,可显著提高基因递送和沉默的效果。体外成像检查表明,应用 12 小时后,荧光标记的 f(Lipo)-RVG29 在脑组织内的沉积增加。此外,体内递送携带 siRNA 的 f(Lipo)-RVG29 大大抑制了脑组织内 BACE1 在 mRNA 和蛋白质水平上的表达。我们的研究结果表明,所开发的非病毒载体是一种很有前景的基因载体系统,它结合了模拟病毒的 RVG29 配体与模仿细胞膜天然脂质双层的生物启发脂质体的协同效应,可用于脑靶向 RNAi 治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
8.60%
发文量
951
审稿时长
72 days
期刊介绍:
The International Journal of Pharmaceutics is the third most cited journal in the "Pharmacy & Pharmacology" category out of 366 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


