Formoterol Reduces the Pro-Inflammatory Phenotype by Enhancing the Activity of Glutaminase in Monocyte-Derived Macrophages in the CVB3-Induced Viral Myocarditis
Abstract
Background
Viral myocarditis (VMC) plays a significant role in heart failure, and there is currently a shortage of available targeted treatments. Macrophage phenotype and function are closely associated with the beta-2 adrenergic receptor (β2-AR).
Method
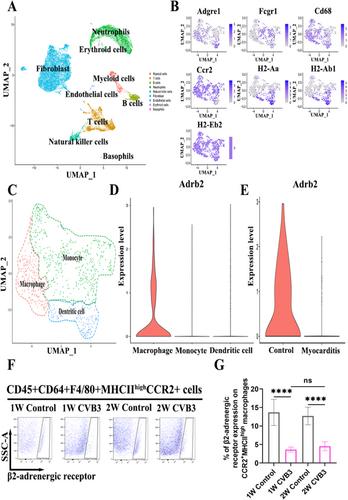
This research employed a BALB/c mouse model of VMC generated using Coxsackievirus B3 (CVB3), and the β2-AR agonist formoterol was administered as treatment. A bioinformatic analysis was conducted to identify the β2-AR in CCR2+MHCIIhigh monocyte-derived macrophages (MoMFs). Echocardiography and histopathological assessments were utilized to evaluate cardiac function and inflammation. The enzymatic activity of glutaminase (GLS) was quantified. Flow cytometry was employed to characterize the phenotype and function of the macrophages.
Result
Our study revealed that formoterol treatment effectively mitigated cardiac inflammation and fibrosis, improved cardiac function, and prolonged survival compared to the VMC group. Formoterol reduced the infiltration of CCR2+MHCIIhigh MoMFs in the heart, inhibited M1 phenotypic expression and activity, and reduced the percentage of Ly6Chigh monocytes in circulation. Additionally, formoterol stimulated M2 phenotypic expression and activity and increased the percentage of Ly6Clow monocytes in circulation. Additionally, the combination of NICB3344, a C-C motif chemokine receptor 2 inhibitor, with formoterol did not exhibit synergistic effects on reducing cardiac pathological scores or enhancing cardiac function. In vitro studies involving the use of lipopolysaccharide (LPS)-induced bone marrow-derived macrophages, revealed the ability of formoterol to suppress the M1 phenotype and functions induced by LPS while promoting the M2 phenotype and functions. Nevertheless, the observed effects were negated by the introduction of the GLS inhibitor BPTES.
Conclusion
Formoterol potentially serves as a significant metabolic regulator in the differentiation process of cardiac MoMFs, influencing this process by controlling GLS activity. Targeting β2-AR exhibits potential as an effective approach for managing VMC. It is essential to acknowledge that these findings were derived under specific experimental conditions, with the current conclusions predominantly based on animal models. Future research is necessary to further investigate the feasibility of formoterol in clinical practice.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


