Dynamic simulation-driven analysis of cadmium, nickel, cobalt, and iron adsorption mechanisms in zeolite LTA synthesized from bentonite
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
A practical and cost-effective method was successfully developed for synthesizing high-performance zeolite LTA from bentonite clay by fine-tuning activation steps and crystallization parameters. The optimal synthesis conditions and crystallization mechanism were investigated. The synthesized zeolites were characterized using XRD, FTIR, and SEM-EDS techniques. The results highlight the significant influence of factors such as crystallization temperature, duration, and the effect of sodium hydroxide concentration on the formation of zeolites. Optimal conditions set at a crystallization temperature of 97 °C, duration of 24 h, and NaOH concentration of 4M yielded pure zeolite LTA, boasting high crystallinity levels. Achieving a peak crystallinity of 82 %. The obtained zeolite LTA showed an exceptional Cd (II) ion exchange capacity. A mechanism involving adsorption of Cd2⁺, Ni2⁺, Co2⁺, and Fe2⁺ ions in zeolite LTA at the α and β-cages has been proposed using dynamic simulation. This mechanism supports all experimental results, in particular for LTA- Cd2⁺, Cd2⁺ ions are predominantly distributed in both α and β-cages, with a denser distribution in the α-cages, indicating a strong preference for these sites due to their geometric and electronic environment. The resulted zeolite LTA demonstrated ability for successful Cd (II) removal, affirming its utility as an efficient material in environmental remediation industries.
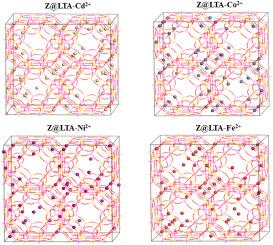
膨润土合成的沸石 LTA 对镉、镍、钴和铁的吸附机理的动态模拟分析
通过对活化步骤和结晶参数进行微调,成功开发出了一种从膨润土中合成高性能沸石 LTA 的实用且具有成本效益的方法。研究了最佳合成条件和结晶机理。利用 XRD、FTIR 和 SEM-EDS 技术对合成的沸石进行了表征。结果表明,结晶温度、持续时间和氢氧化钠浓度等因素对沸石的形成有重大影响。在结晶温度为 97 °C、持续时间为 24 小时、NaOH 浓度为 4M 的最佳条件下,产生了高结晶度的纯沸石 LTA。最高结晶度达到 82%。所获得的沸石 LTA 具有优异的镉(II)离子交换能力。利用动态模拟提出了沸石 LTA α 和 β 笼中 Cd2⁺、Ni2⁺、Co2⁺ 和 Fe2⁺离子的吸附机理。该机制支持所有的实验结果,特别是对于 LTA- Cd2⁺,Cd2⁺ 离子主要分布在 α 和 β 笼中,在 α 笼中分布更密集,这表明由于其几何和电子环境,Cd2⁺ 离子对这些位点有强烈的偏好。所制备的沸石 LTA 具有成功去除镉(II)的能力,这肯定了它在环境修复行业中作为一种高效材料的实用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


