Metalation of porphyrin units in a porous organic polymer stabilizing its anodic cycling performance in lithium-ion battery
IF 8.1
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Organic materials have attracted tremendous interest as electrodes materials for lithium-ion battery, however, they still suffer from intractable problems including inherent low electronic conductivity, poor cycling stability and high solubility in electrolyte etc. Herein, a porphyrin-based porous organic polymer (POP) is employed to serve as anode material by virtue of its good insolubility, unique π-π interaction and abundant active sites. The POP electrode exhibits a high specific capacity of 584 mAh g−1 at 100 mA g−1 originated from fast redox activity while unfortunately undergoes a severe capacity fading during long-term cycles. It is found that the metalation of porphyrin moiety in POP by Ni2+ (Ni-POP) enables an excellent cycling stability of 380 mAh g−1 with a capacity retention of 99 % for 500 cycles. We revealed that porphyrin unit is decomposed from the cleavage of the C-N/C=N bonds in porphyrin units, while Ni coordination stabilizes porphyrin units during fast redox process as affirmed by ex situ X-ray photoelectron spectroscopy. The easy fabrication, low-cost and excellent performance make Ni-POP great potential as anode material for lithium-ion battery.
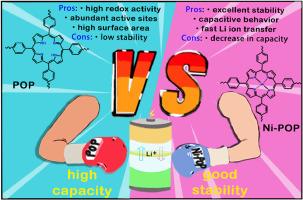
多孔有机聚合物中卟啉单元的金属化可稳定其在锂离子电池中的阳极循环性能
有机材料作为锂离子电池的电极材料引起了人们的极大兴趣,然而,它们仍然存在着难以解决的问题,包括固有的低电子传导性、循环稳定性差以及在电解液中的高溶解性等。在本文中,卟啉基多孔有机聚合物(POP)凭借其良好的不溶性、独特的π-π相互作用和丰富的活性位点被用作负极材料。该 POP 电极在 100 mA g-1 的条件下可显示出 584 mAh g-1 的高比容量,这源于其快速的氧化还原活性。研究发现,用 Ni2+(Ni-POP)对 POP 中的卟啉分子进行金属化处理,可实现 380 mAh g-1 的优异循环稳定性,500 次循环的容量保持率为 99%。原位 X 射线光电子能谱证实,卟啉单元中的 C-N/C=N 键裂解分解了卟啉单元,而 Ni 配位在快速氧化还原过程中稳定了卟啉单元。Ni-POP 易于制造、成本低廉、性能优异,因此极有可能成为锂离子电池的负极材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Power Sources
工程技术-电化学
CiteScore
16.40
自引率
6.50%
发文量
1249
审稿时长
36 days
期刊介绍:
The Journal of Power Sources is a publication catering to researchers and technologists interested in various aspects of the science, technology, and applications of electrochemical power sources. It covers original research and reviews on primary and secondary batteries, fuel cells, supercapacitors, and photo-electrochemical cells.
Topics considered include the research, development and applications of nanomaterials and novel componentry for these devices. Examples of applications of these electrochemical power sources include:
• Portable electronics
• Electric and Hybrid Electric Vehicles
• Uninterruptible Power Supply (UPS) systems
• Storage of renewable energy
• Satellites and deep space probes
• Boats and ships, drones and aircrafts
• Wearable energy storage systems
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


