3D bioprinting of thick core–shell vascularized scaffolds for potential tissue engineering applications
IF 5.8
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
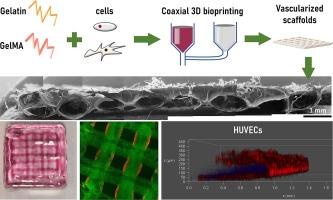
The promise of tissue engineering in developing functional, living, 3D thick structures has been limited due to the constraints of nutrient and oxygen delivery through diffusion. Although advancements in additive manufacturing approaches have enhanced the fidelity and complexity of 3D (bio)printed constructs, the vascularization of such scaffolds is less investigated. Here, we have leveraged extrusion-based 3D bioprnting of core/shell constructs to develop millimeter-thick scaffolds with embedded microvasculature for potential soft tissue repair. Composites of methacrylated gelatin (GelMA) and gelatin have been used for this purpose. A systematic approach was used to investigate the effect of parameters, such as material and photoinitiator concentrations, and photocuring time, on the properties of constructs. Results have shown the structures have Young’s modulus close to the soft tissues. 3D bioprinting parameters were optimized so that the printing and photo crosslinking procedures did not negatively affect the cell viability. It was also observed that a continuous hollow inner core could be successfully printed within the scaffolds, which upon incorporation of endothelial cells during the 3D bioprinting process, could form micro-vessels embedded in the constructs. Together, our results demonstrate the significant potential of the proposed approach for developing thick vascularized tissue-engineered scaffolds suitable for soft tissue engineering.
厚核壳血管化支架的三维生物打印技术在组织工程中的潜在应用
由于通过扩散输送营养和氧气的限制,组织工程在开发功能性、活体三维厚结构方面的前景一直很有限。虽然增材制造方法的进步提高了三维(生物)打印结构的保真度和复杂性,但对此类支架的血管化研究较少。在这里,我们利用基于挤压技术的三维生物芯/壳结构,开发出了具有嵌入式微血管的毫米厚支架,用于潜在的软组织修复。为此,我们使用了甲基丙烯酸明胶(GelMA)和明胶的复合材料。该研究采用了一种系统方法来研究材料、光引发剂浓度和光固化时间等参数对构建物特性的影响。结果表明,这些结构的杨氏模量接近软组织。对三维生物打印参数进行了优化,使打印和光交联过程不会对细胞活力产生负面影响。我们还观察到,支架内可以成功打印出连续的中空内核,在三维生物打印过程中加入内皮细胞后,可形成嵌入构建物的微血管。总之,我们的研究结果表明,所提出的方法在开发适用于软组织工程的厚血管组织工程支架方面具有巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


