Decellularized adipose matrix hydrogel-based in situ delivery of antagomiR-150-5p for rat abdominal aortic aneurysm therapy
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
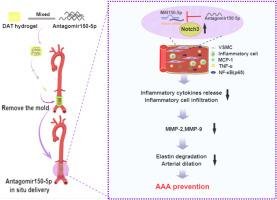
Abdominal aortic aneurysm (AAA) is a progressive aortic disease featured by inflammation, vascular smooth muscle cells (VSMCs) depletion, and elastin degradation. MicroRNAs were related to AAA formation, which bring the approach for precise and targeted drug therapy for AAA. We developed a new strategy based on decellularized adipose matrix (DAM) hydrogel immobilized on the adventitia to release antagomiR-150-5p for preventing the AAA development. In this study, Cacl2-induced and elastase-induced rat AAA models were established. We found that miR-150-5p was upregulated while Notch3 was downregulated in two rat AAA models. Then a mold was designed for shaping hydrogel for miR-150-5p delivery around the abdominal aorta. Interestingly, inhibition of miR-150-5p in AAA by local release of antagomiR-150-5p with DAM hydrogel significantly prevented aortic dilation and elastin degradation. Moreover, inflammatory cell infiltration, the expression of inflammatory cytokines (MCP-1, TNF-α, and NF-κB (p65)), and matrix metalloproteinases (MMP-2, MMP-9) were increased while Notch3 and α-SMA were decreased in rat AAA, which can be attenuated by antagomiR-150-5p treatment. In VSMCs with TNF-α stimulation, we further demonstrated that inhibition of miR-150-5p downregulated NF-κB (p65), MMP-2, and MMP-9 and upregulated elastin via Notch3. This work presents a translational potential strategy for AAA repair via DAM hydrogel sustained release of antagomiR-150-5p, and highlights the mechanism of miR-150-5p during AAA progression by regulating Notch3.
基于脱细胞脂肪基质水凝胶的原位递送抗噬菌体-150-5p,用于治疗大鼠腹主动脉瘤
腹主动脉瘤(AAA)是一种以炎症、血管平滑肌细胞(VSMC)耗竭和弹性蛋白降解为特征的进行性主动脉疾病。微RNA与AAA的形成有关,这为AAA的精确靶向药物治疗提供了方法。我们开发了一种新策略,基于固定在血管前壁的脱细胞脂肪基质(DAM)水凝胶来释放抗组蛋白R-150-5p,从而预防AAA的发生。本研究建立了 Cacl2 诱导和弹性蛋白酶诱导的大鼠 AAA 模型。我们发现,在两种大鼠 AAA 模型中,miR-150-5p 上调而 Notch3 下调。然后,我们设计了一种水凝胶模具,用于在腹主动脉周围输送 miR-150-5p。有趣的是,通过 DAM 水凝胶局部释放抗 miR-150-5p 抑制 AAA 中的 miR-150-5p,能显著防止主动脉扩张和弹性蛋白降解。此外,在大鼠 AAA 中,炎性细胞浸润、炎性细胞因子(MCP-1、TNF-α 和 NF-κB (p65))和基质金属蛋白酶(MMP-2、MMP-9)的表达增加,而 Notch3 和 α-SMA 的表达减少,这都可以通过 antagomiR-150-5p 的处理得到缓解。在 TNF-α 刺激的 VSMCs 中,我们进一步证实抑制 miR-150-5p 可下调 NF-κB(p65)、MMP-2 和 MMP-9,并通过 Notch3 上调弹性蛋白。这项研究提出了一种通过 DAM 水凝胶持续释放抗 miR-150-5p 来修复 AAA 的潜在转化策略,并强调了 miR-150-5p 通过调控 Notch3 在 AAA 进展过程中的作用机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
文献相关原料
公司名称
产品信息
索莱宝
CaCl2
索莱宝
Tissue DNA Extraction Kit
上海源叶
Elastase
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


