MSCs-derived ECM functionalized hydrogel regulates macrophage reprogramming for osteoarthritis treatment by improving mitochondrial function and energy metabolism
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Osteoarthritis (OA) is a degenerative disease that affects the entire joint, with synovial inflammation being a major pathological feature. Macrophages, as the most abundant immune cells in the synovium, have an M1/M2 imbalance that is closely related to the occurrence and development of OA. Mesenchymal stem cells (MSCs) have been shown to effectively suppress inflammation in the treatment of OA, but they still pose issues such as immune rejection and tumorigenicity. The extracellular matrix (ECM), as a major mediator of MSCs' immunoregulatory effects, offers a cell-free therapy to circumvent these risks. In this study, we developed an ECM-functionalized hydrogel by combining MSC-derived ECM with gelatin methacryloyl (GelMA). To enhance the immunomodulatory potential of MSCs, we pre-stimulated MSCs with the inflammatory factor interleukin-6 (IL-6) present in OA. In vitro results showed that the ECM-functionalized hydrogel promoted M2 macrophage polarization and inhibited the expression of various inflammatory genes, strongly indicating the hydrogel's powerful immunoregulatory capabilities. In an in vivo rat OA model, the ECM-functionalized hydrogel significantly reduced synovial inflammation and cartilage matrix degradation, alleviating the progression of OA. Furthermore, we utilized proteomics and transcriptomics analysis to reveal that the hydrogel accomplished macrophage metabolic reprogramming by regulating mitochondrial function and energy metabolism, thereby reducing inflammation. These findings suggest that the ECM-functionalized hydrogel is a promising biomaterial-based strategy for treating OA by targeting key pathological mechanisms.
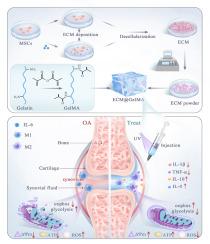
间充质干细胞衍生的 ECM 功能化水凝胶通过改善线粒体功能和能量代谢调节巨噬细胞重编程,从而治疗骨关节炎
骨关节炎(OA)是一种影响整个关节的退行性疾病,滑膜炎症是其主要病理特征。巨噬细胞作为滑膜中最丰富的免疫细胞,其M1/M2失衡与OA的发生和发展密切相关。间充质干细胞(MSCs)已被证明能有效抑制炎症,用于治疗 OA,但仍存在免疫排斥和致瘤性等问题。细胞外基质(ECM)作为间充质干细胞免疫调节作用的主要介质,为规避这些风险提供了一种无细胞疗法。在这项研究中,我们将间叶干细胞衍生的 ECM 与甲基丙烯酰明胶(GelMA)结合,开发出了一种 ECM 功能化水凝胶。为了提高间充质干细胞的免疫调节潜力,我们预先用 OA 中的炎症因子白细胞介素-6(IL-6)刺激间充质干细胞。体外实验结果表明,ECM功能化水凝胶促进了M2巨噬细胞的极化,抑制了各种炎症基因的表达,有力地证明了水凝胶强大的免疫调节能力。在体内大鼠 OA 模型中,ECM 功能化水凝胶显著减轻了滑膜炎症和软骨基质降解,缓解了 OA 的恶化。此外,我们利用蛋白质组学和转录组学分析发现,水凝胶通过调节线粒体功能和能量代谢实现了巨噬细胞代谢重编程,从而减轻了炎症。这些研究结果表明,ECM 功能化水凝胶是针对关键病理机制治疗 OA 的一种很有前景的生物材料策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


