Unraveling biomolecules, antidiabetic and antioxidants properties of DelitesTM via pharmacoinformatics and in vitro investigation
Pharmacological Research - Modern Chinese Medicine
Pub Date : 2024-11-20
DOI:10.1016/j.prmcm.2024.100551
引用次数: 0
Abstract
Introduction
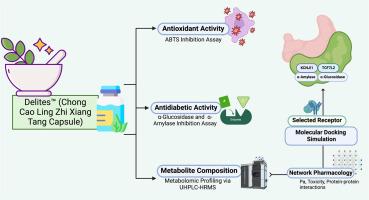
Type 2 diabetes (T2D) remains a global health burden characterized by insulin resistance and chronic hyperglycemia, often exacerbated by oxidative stress, leading to severe complications. Despite the efficacy of pharmacological treatments such as metformin, their side effects and costs highlight the need for alternative therapies. Delites™ (Chong Cao Ling Zhi Xiang Tang), a multi-herbal formulation, presents a promising solution with its bioactive compounds including Apocynin, Curcumin, and Quercetin, which are hypothesized to target T2D pathways.
Methods
This study employed pharmacoinformatics, in vitro assays, and molecular dynamics simulations to evaluate the antidiabetic and antioxidative properties of Delites™. Metabolomic profiling using Ultra-High-Performance Liquid Chromatography coupled with High-Resolution Mass Spectrometry identified active compounds, while in silico docking analyzed interactions with T2D-related proteins (e.g., KCNJ11, TCF7L2). Enzyme inhibition assays measured alpha-glucosidase and alpha-amylase activity, and antioxidant potential was assessed using ABTS inhibition.
Results
Delites™ demonstrated significant enzyme inhibition (EC50 < metformin), strong binding affinity to T2D proteins (e.g., Sachaliside 2: -9.4 kcal/mol with TCF7L2), and antioxidant activity comparable to Trolox (EC50: 54.44 mg/mL). Molecular dynamics confirmed stable interactions of its compounds with target proteins, while network pharmacology highlighted multi-target potential against diabetes-related pathways.
Discussion
The findings underline Delites™ as a multi-target therapeutic candidate for T2D management. Its ability to inhibit carbohydrate-hydrolyzing enzymes, interact strongly with key proteins, and mitigate oxidative stress positions it as a holistic alternative. However, further clinical trials are essential to validate these promising in vitro and in silico results, particularly its long-term efficacy and safety.
通过药物信息学和体外研究揭示得利特TM的生物分子、抗糖尿病和抗氧化特性
导言2型糖尿病(T2D)仍然是一个全球性的健康负担,其特点是胰岛素抵抗和慢性高血糖,往往因氧化应激而加剧,导致严重的并发症。尽管二甲双胍等药物治疗具有一定的疗效,但其副作用和成本凸显了对替代疗法的需求。Delites™(冲草灵芝香汤)是一种多草药制剂,其生物活性化合物包括阿朴昔宁、姜黄素和槲皮素,据推测可靶向治疗 T2D 的途径,是一种很有前景的解决方案。利用超高效液相色谱法和高分辨质谱法进行的代谢组学分析确定了活性化合物,而硅学对接分析了与 T2D 相关蛋白(如 KCNJ11、TCF7L2)的相互作用。酶抑制试验测定了α-葡萄糖苷酶和α-淀粉酶的活性,并使用 ABTS 抑制剂评估了抗氧化潜力。结果Delites™ 显示出显著的酶抑制作用(EC50 <;二甲双胍)、与 T2D 蛋白质的强结合亲和力(例如,Sachaliside 2:与 TCF7L2 的结合亲和力为 -9.4 kcal/mol)以及与 Trolox 相当的抗氧化活性(EC50:54.44 mg/mL)。分子动力学证实了其化合物与靶蛋白的稳定相互作用,而网络药理学则强调了针对糖尿病相关通路的多靶点潜力。它能够抑制碳水化合物水解酶,与关键蛋白产生强烈的相互作用,并能减轻氧化应激,是一种全面的替代疗法。然而,要验证这些有前景的体外和硅学结果,特别是其长期疗效和安全性,还需要进一步的临床试验。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


