Metabolic intervention mitochondria nanomotors breakdown redox homeostasis for boosting oxidative stress-dependent antitumor therapy
IF 21.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Aberrant metabolic balance in malignant tumors shapes defensive redox homeostasis and protected tumor cells from oxidative stress damage, consequently impeding clinical transformation process of oxidative stress-dependent anti-tumor therapies represented by chemodynamic therapy and immunotherapy. Herein, a rational designed mitochondria nanomotor was developed by coating a GSH-responsive functional Pt(IV) prodrug layer DP on magnetic iron oxide nanoparticles (MN), which can thoroughly breakdown redox homeostasis by metabolic intervention strategy. Specifically, DP loading two dichloroacetic acid (DCA) axial ligands was stimuli-responsively reduced into Pt(II) and DCA molecules in highly reductive tumor cells, accompanied with glutathione elimination and oxidative stress counteraction weakening. Subsequently, DCA increased pyruvate influx into the mitochondria by pyruvate dehydrogenase activation and enduringly elevated oxidative phosphorylation level, breaking the tumor redox homeostasis thoroughly, contributing to 7.5-fold amplifying hydrogen peroxide production and sensitizing chemodynamic therapy mediated by MN, finally resulting in inspiring 89.5% tumor suppression rate on triple negative breast cancer model. In short, this work realized comprehensive and sustainable oxidative stress elevation of the intracellular environment by metabolic intervention strategy and provided an ingenious perspective of augmenting oxidative stress-dependent anti-tumor therapies.
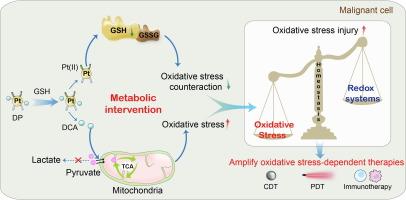
代谢干预线粒体纳米马达打破氧化还原平衡,促进氧化应激依赖性抗肿瘤治疗
恶性肿瘤代谢平衡失调会影响防御性氧化还原平衡,保护肿瘤细胞免受氧化应激损伤,从而阻碍以化学动力疗法和免疫疗法为代表的氧化应激依赖型抗肿瘤疗法的临床转化进程。本文通过在磁性氧化铁纳米粒子(MN)上包覆GSH响应性功能铂(IV)原药层DP,开发了一种合理设计的线粒体纳米马达,可通过代谢干预策略彻底打破氧化还原平衡。具体来说,在高还原性肿瘤细胞中,含有两种二氯乙酸(DCA)轴向配体的DP被刺激性还原成铂(II)和DCA分子,同时谷胱甘肽被消除,氧化应激反应减弱。随后,DCA通过激活丙酮酸脱氢酶增加丙酮酸流入线粒体,并持续升高氧化磷酸化水平,彻底打破了肿瘤的氧化还原平衡,使过氧化氢的产生量增加了7.5倍,对MN介导的化学动力学治疗产生了增敏作用,最终使三阴性乳腺癌模型的抑瘤率达到89.5%。总之,这项工作通过代谢干预策略实现了细胞内环境全面、可持续的氧化应激升高,为增强氧化应激依赖性抗肿瘤疗法提供了一个巧妙的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


