Light-activated polymeric crosslinked nanocarriers as a checkpoint blockade immunoregulatory platform for synergistic tumor therapy
IF 21.1
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Photodynamic therapy (PDT) can enhance immune checkpoint blockade (ICB) antitumor immunity. However, PDT can significantly exacerbate the hypoxic tumor microenvironment and stimulate tumor neovascularization, promoting tumor invasion and metastasis. Camptothecin can inhibit angiogenesis by down-regulating hypoxia-inducible factor 1α (HIF-1α). Therefore, this study proposed to combine camptothecin with PDT for the first time to alleviate the disadvantage of PDT, and play its dual role of chemotherapy and antiangiogenesis. Here, a light-activated nanocarrier crosslinked the anti-PD-L1, photosensitizer, and camptothecin prodrug mildly with a thioketal bond for checkpoint blockade immunoregulation was designed. Firstly, photosensitizer-induced PDT and immunogenic cell death effect significantly increase T cell infiltration (33.3 % CD8+ increase), enhancing ICB antitumor immunity. Secondly, the antiangiogenic effect of camptothecin was beneficial for alleviating hypoxic tumor microenvironment exacerbated by PDT (HIF-1α expression decreased in tumor cells). Thirdly, light-activated release facilitates tumor accumulation (3.22 times) and controlled drug release. Thus, the immune checkpoint blockade combined with PDT and an antiangiogenic therapy of camptothecin creates a positive feedback co-delivery platform that exemplifies cascaded synergistic tumor therapy by checkpoint blockade immunoregulation. Besides, it also introduces a new strategy for combining small molecule drugs with macromolecules like proteins to treat various diseases.
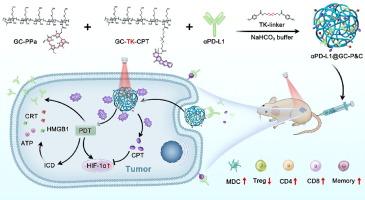
光活化聚合物交联纳米载体作为检查点阻断免疫调节平台用于肿瘤协同治疗
光动力疗法(PDT)可以增强免疫检查点阻断(ICB)的抗肿瘤免疫力。然而,光动力疗法会明显加剧缺氧的肿瘤微环境,刺激肿瘤新生血管,促进肿瘤的侵袭和转移。喜树碱可通过下调缺氧诱导因子1α(HIF-1α)抑制血管生成。因此,本研究首次提出将喜树碱与光动力疗法相结合,以缓解光动力疗法的缺点,发挥其化疗和抗血管生成的双重作用。本文设计了一种将抗PD-L1、光敏剂和喜树碱原药以硫酮键温和交联的光激活纳米载体,用于检查点阻断免疫调节。首先,光敏剂诱导的PDT和免疫原性细胞死亡效应显著增加了T细胞浸润(CD8+增加33.3%),增强了ICB的抗肿瘤免疫力。其次,喜树碱的抗血管生成作用有利于缓解因 PDT 而恶化的缺氧肿瘤微环境(肿瘤细胞中 HIF-1α 的表达减少)。第三,光激活释放有利于肿瘤积聚(3.22 倍)和药物的可控释放。因此,免疫检查点阻断与光动力疗法和喜树碱抗血管生成疗法相结合,创建了一个正反馈联合给药平台,体现了检查点阻断免疫调节的级联协同肿瘤治疗。此外,它还介绍了一种将小分子药物与蛋白质等大分子结合治疗各种疾病的新策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today
工程技术-材料科学:综合
CiteScore
36.30
自引率
1.20%
发文量
237
审稿时长
23 days
期刊介绍:
Materials Today is the leading journal in the Materials Today family, focusing on the latest and most impactful work in the materials science community. With a reputation for excellence in news and reviews, the journal has now expanded its coverage to include original research and aims to be at the forefront of the field.
We welcome comprehensive articles, short communications, and review articles from established leaders in the rapidly evolving fields of materials science and related disciplines. We strive to provide authors with rigorous peer review, fast publication, and maximum exposure for their work. While we only accept the most significant manuscripts, our speedy evaluation process ensures that there are no unnecessary publication delays.
文献相关原料
公司名称
产品信息
阿拉丁
4-dimethylaminopyridine (DMAP)
阿拉丁
lithium aluminium hydride
阿拉丁
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC)
阿拉丁
triphosgene
阿拉丁
thioglycolic acid
阿拉丁
(S)-(+)-Camptothecin
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


