Correlation between activation energy and reaction temperature as observed in thermal analysis kinetics
IF 3.1
2区 化学
Q2 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
The activation energies (E) and temperatures (T) of a variety of chemical reactions are analyzed to reveal the following correlation: E(kJ/mol)=(35±12)+(0.46±0.04)T(°C). The correlation is statistically significant having the Pearson's coefficient of linear correlation 0.873. Consequently, 76 % of the observed variation in the activations energy is caused by the actual relationship between E and T, whereas only 24 % is attributed to chance. A statistically similar correlation is revealed for crystallization of glasses. In practice, such correlations can be used to estimate the initial E values in nonlinear optimization of kinetic models or to quickly check whether the experimental activation energies disagree excessively with the expected values. An attempt is made to identify the physical origins of the correlation.
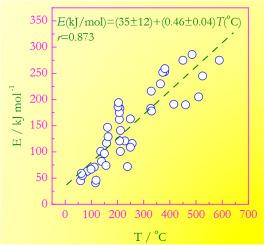
热分析动力学中观察到的活化能与反应温度之间的相关性
通过分析各种化学反应的活化能 (E) 和温度 (T),可以发现以下相关性:E(kJ/mol)=(35±12)+(0.46±0.04)T(°C).该相关性具有显著的统计学意义,皮尔逊线性相关系数为 0.873。因此,在观察到的活化能变化中,有 76% 是由 E 和 T 之间的实际关系引起的,只有 24% 是偶然因素。在统计上,玻璃的结晶也有类似的相关性。在实践中,这种相关性可用于估计动力学模型非线性优化中的初始 E 值,或用于快速检查实验活化能是否与预期值相差过大。本文试图找出相关性的物理根源。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Thermochimica Acta
化学-分析化学
CiteScore
6.50
自引率
8.60%
发文量
210
审稿时长
40 days
期刊介绍:
Thermochimica Acta publishes original research contributions covering all aspects of thermoanalytical and calorimetric methods and their application to experimental chemistry, physics, biology and engineering. The journal aims to span the whole range from fundamental research to practical application.
The journal focuses on the research that advances physical and analytical science of thermal phenomena. Therefore, the manuscripts are expected to provide important insights into the thermal phenomena studied or to propose significant improvements of analytical or computational techniques employed in thermal studies. Manuscripts that report the results of routine thermal measurements are not suitable for publication in Thermochimica Acta.
The journal particularly welcomes papers from newly emerging areas as well as from the traditional strength areas:
- New and improved instrumentation and methods
- Thermal properties and behavior of materials
- Kinetics of thermally stimulated processes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


