Efficient ranitidine removal using Si3N4-ZVI/PAA system: Mechanistic insights and environmental implications
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
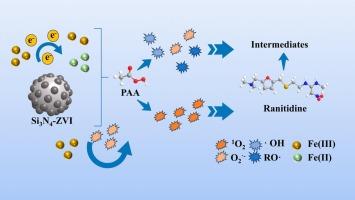
In this study, silicon nitride-zero-valent iron (Si3N4-ZVI) powders were obtained through post-milling and employed as a catalyst to activate peracetic acid (PAA) for the removal of Ranitidine (RAN). The system achieved an impressive 90.4 % of degradation efficiency, with a pseudo-first-order kinetic reaction constant 11.3 times higher than that of the ZVI/PAA system. Quenching experiments and Electron Paramagnetic Resonance (EPR) analysis confirmed that both radical and non-radical processes are involved in RAN degradation within the Si3N4-ZVI/PAA system. Moreover, the identified ten degradation products are less toxic compared to the byproducts from alternative oxidation methods reported by prior literature. The proposed degradation pathway of RAN includes C![]() S cleavage, oxygenation/hydroxylation demethylation, and denitrification. Furthermore, the Si3N4-ZVI/PAA system showed effectively in treating RAN-contaminated surface water, artificial wastewater, and dissolved organic matter, demonstrating its great ability in treating diverse organic-contaminated water sources. In conclusion, results of this study suggest that the Si3N4-ZVI/PAA system possess high pollutant removal efficiency, broad applicability, and less environmental and health risks.
S cleavage, oxygenation/hydroxylation demethylation, and denitrification. Furthermore, the Si3N4-ZVI/PAA system showed effectively in treating RAN-contaminated surface water, artificial wastewater, and dissolved organic matter, demonstrating its great ability in treating diverse organic-contaminated water sources. In conclusion, results of this study suggest that the Si3N4-ZVI/PAA system possess high pollutant removal efficiency, broad applicability, and less environmental and health risks.
利用 Si3N4-ZVI/PAA 系统高效去除雷尼替丁:机理认识和环境影响
本研究通过后研磨获得了氮化硅-零价铁(Si3N4-ZVI)粉末,并将其用作催化剂激活过乙酸(PAA)以去除雷尼替丁(RAN)。该系统的降解效率达到了惊人的 90.4%,其假一阶动力学反应常数是 ZVI/PAA 系统的 11.3 倍。淬火实验和电子顺磁共振(EPR)分析证实,在 Si3N4-ZVI/PAA 系统中,自由基和非自由基过程都参与了 RAN 降解。此外,与之前文献报道的其他氧化方法产生的副产品相比,已确定的十种降解产物毒性较低。建议的 RAN 降解途径包括 CS 裂解、氧化/羟化脱甲基化和脱硝。此外,Si3N4-ZVI/PAA 系统还能有效处理受 RAN 污染的地表水、人工废水和溶解有机物,显示出其在处理各种有机物污染水源方面的巨大能力。总之,本研究的结果表明,Si3N4-ZVI/PAA 系统具有较高的污染物去除效率、广泛的适用性以及较低的环境和健康风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


