Kinetic and mechanistic insights into the evolution of sulfur-centered radicals in transition-metal-activated bisulfite processes
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
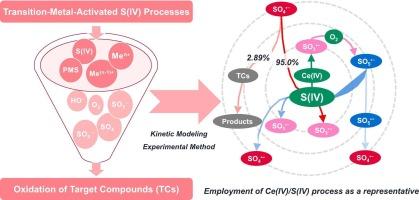
Transition-metal-activated S(IV) processes hold excellent application potential for decontamination. However, the underlying redox chemistry remains largely obscure, impeding the advancement and practical implementation of these processes. Hence, there is an urgent need to explore the intricate redox chemistry to facilitate the development and application of transition-metal-activated S(IV) processes. In this study, we selected the Ce(IV)/S(IV) process as a representative system and employed kinetic modeling to explore the evolution of sulfur-centered radicals (i.e., SO3•−, SO5•−, and SO4•−) under acidic conditions. The oxidation of S(IV) by various oxidants (i.e., Ce(IV), SO5•−, and SO4•−) could produce SO3•−, which was regarded as a critical sulfur-centered radical for SO4•− production. Despite the efficient transformation of SO5•− into SO4•−, 95.0% of the generated SO4•− was rapidly consumed by S(IV) due to its high reactivity towards S(IV). The yield and utilization of SO4•− were further investigated using kinetic modeling and experimental methods. The yield of SO4•− was independent of target compounds (TCs), whereas the utilization of SO4•− by TCs depended on the captured capacity of TCs for SO4•−. Moreover, controlling a low dosage of S(IV) significantly improved both the yield and utilization of SO4•− in the Ce(IV)/S(IV) process. This work provides valuable insights into the redox chemistry and kinetic behavior of transition-metal-activated S(IV) processes, thereby guiding the development and application of transition-metal-activated S(IV) processes in decontamination.
过渡金属活化亚硫酸氢盐过程中硫为中心的自由基演变的动力学和机械学见解
过渡金属活化 S(IV)工艺在净化方面具有巨大的应用潜力。然而,其背后的氧化还原化学原理在很大程度上仍然模糊不清,阻碍了这些工艺的发展和实际应用。因此,迫切需要探索复杂的氧化还原化学,以促进过渡金属活化 S(IV)工艺的开发和应用。在本研究中,我们选择了 Ce(IV)/S(IV) 过程作为代表性体系,并采用动力学模型来探索酸性条件下以硫为中心的自由基(即 SO3--、SO5--和 SO4--)的演化过程。各种氧化剂(即 Ce(IV)、SO5--和 SO4--)对 S(IV)的氧化可产生 SO3--,SO3--被认为是产生 SO4--的关键硫心自由基。尽管 SO5--能高效转化为 SO4--,但由于其对 S(IV)的高反应性,生成的 SO4--有 95.0% 被 S(IV)迅速消耗。我们利用动力学模型和实验方法进一步研究了 SO4--的产率和利用率。SO4--的产量与目标化合物(TCs)无关,而TCs对SO4--的利用则取决于TCs对SO4--的捕获能力。此外,在 Ce(IV)/S(IV) 工艺中,控制 S(IV) 的低用量可显著提高 SO4-- 的产率和利用率。这项研究为了解过渡金属活化 S(IV)过程的氧化还原化学和动力学行为提供了宝贵的见解,从而指导了过渡金属活化 S(IV)过程在净化领域的开发和应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


