Enhanced removal of ciprofloxacin antibiotic using agricultural byproduct-derived biochars: From studies on adsorption kinetic, isotherm and thermodynamic to explore mechanistic insights into the removal pathway
IF 5.5
3区 工程技术
Q1 ENGINEERING, CHEMICAL
Journal of the Taiwan Institute of Chemical Engineers
Pub Date : 2024-11-28
DOI:10.1016/j.jtice.2024.105846
引用次数: 0
Abstract
Background
Ciprofloxacin (CIP) antibiotic, classified as an emerging organic contaminant, has caused an adverse impact on the ecological environment due to its persistence.
Methods
In this study, CIP was adsorbed on a novel nanocomposite using lanthanum ferrite spinel nanoparticles (LaFe2O4 NPs) dispersed on litchi shell-derived biochar supporter (BLF) with various loading ratios. The adsorption tests were conducted in batch mode to thoroughly investigate operational parameter effects. In addition, the adsorption kinetics, isotherms, and thermodynamics of CIP onto BLF at various temperatures were systematically studied. The as-synthesized adsorbent was thoroughly characterisized. Finally, CIP adsorption mechanisms onto BLF were revealed.
Significant findings
Our findings showed that the pristine biochar (PBC) was loaded by LaFe2O4, which resulted in more thorough carbonization, enhanced aromaticity, hydrophobicity, porosity, and enriched surface functional groups. The CIP adsorption onto BLF was the highest under optimal operational conditions of solution pH of 5.0, 3.0 g/L biochar dosage, and 50 mg/L initial CIP concentration. The pseudo-second order model best matched the CIP adsorption kinetics with high correlation coefficients of 0.9653–0.9939. The Langmuir model better described the adsorption behaviors of CIP on BLF, with a maximum adsorption capacity of 36.5 mg/g, which was greater than that on PBC by about 1.5 times. CIP adsorption on the BLF exhibited a spontaneous and endothermic nature. The primary mechanisms of CIP adsorption on the BLF were H-bonding and π-π interaction. In particular, La and Fe constituents in BLF functionally enhanced the CIP adsorption via the surface complexation mechanism. These findings illustrated that the BLF was a novel, feasible, and promising adsorbent for the effective removal of CIP antibiotics from water.
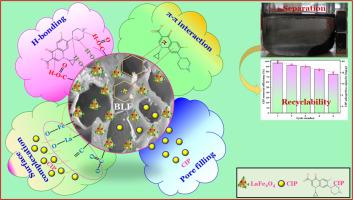
利用源自农副产品的生物炭提高环丙沙星抗生素的去除率:从吸附动力学、等温线和热力学研究探索去除途径的机理见解
背景环丙沙星(Ciprofloxacin,CIP)抗生素被列为一种新出现的有机污染物,因其持久性对生态环境造成了不利影响。方法本研究使用分散在荔枝壳生物炭支持物(BLF)上的镧铁尖晶石纳米颗粒(LaFe2O4 NPs),以不同的负载率在新型纳米复合材料上吸附了 CIP。吸附试验以批处理模式进行,以深入研究操作参数的影响。此外,还系统研究了不同温度下 CIP 在 BLF 上的吸附动力学、等温线和热力学。对合成的吸附剂进行了全面的表征。重要发现我们的研究结果表明,LaFe2O4 在原始生物炭(PBC)上的负载使其碳化更彻底,芳香度、疏水性、孔隙率得到增强,表面官能团也更加丰富。在溶液 pH 值为 5.0、生物炭用量为 3.0 g/L、CIP 初始浓度为 50 mg/L 的最佳操作条件下,BLF 上的 CIP 吸附量最高。伪二阶模型与 CIP 吸附动力学最匹配,相关系数高达 0.9653-0.9939。Langmuir 模型更好地描述了 CIP 在 BLF 上的吸附行为,其最大吸附容量为 36.5 mg/g,比在 PBC 上的吸附容量高出约 1.5 倍。CIP 在 BLF 上的吸附表现出自发的内热性质。CIP 在 BLF 上的主要吸附机理是 H 键作用和 π-π 相互作用。特别是,BLF 中的 La 和 Fe 成分通过表面络合机制增强了对 CIP 的吸附。这些研究结果表明,BLF 是一种新型、可行且有前景的吸附剂,可有效去除水中的 CIP 抗生素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.10
自引率
14.00%
发文量
362
审稿时长
35 days
期刊介绍:
Journal of the Taiwan Institute of Chemical Engineers (formerly known as Journal of the Chinese Institute of Chemical Engineers) publishes original works, from fundamental principles to practical applications, in the broad field of chemical engineering with special focus on three aspects: Chemical and Biomolecular Science and Technology, Energy and Environmental Science and Technology, and Materials Science and Technology. Authors should choose for their manuscript an appropriate aspect section and a few related classifications when submitting to the journal online.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


