Anticancer activity of the synthetic kusunokinin analogues on human cancer cell lines
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The series of racemic kusunokinin derivatives were synthesized and their cytotoxic activities and cell viability on cancer cells including breast cancer (MCF-7, MDA-MB468), colon cancer (HT-29), cholangiocarcinoma (KKU-M213) and ovarian cancer (A2780) cells were investigated. The results showed that compounds 6aa, 6da, and 6de exhibited growth inhibition against breast cancer (MDA-MB468), cholangiocarcinoma (KKU-M213), colon cancer (HT-29), and ovarian cancer (A2780) cells with IC50 values (μM) 13.77 ± 0.38, 7.94 ± 0.45, and 4.22 ± 0.13 (MDA-MB468); 4.21 ± 0.21, 0.97 ± 0.03, and 0.09 ± 0.02 (KKU-M213); 22.66 ± 0.23, and 15.62 ± 0.06 (HT-29); 13.11 ± 0.37, 11.51 ± 0.43, and 1.87 ± 0.01 (A2780); respectively. Interestingly, a positive control, doxorubicin, showed less cytotoxicity than 6da and 6de on cholangiocarcinoma KKU-M213 and ovarian cancer A2780 cells. Moreover, these three synthetic compounds also exhibited less toxicity than doxorubicin on the normal cells, MMNK-1, Vero and L-929. The binding possibility towards CSF1R, 6de (−11.59 kcal/mol) and trans-(−)-kusunokinin (−11.75 kcal/mol) were similar in both docking energies and docking poses. 6de interacted with Trp550 via π-π stacking in the similar manner with trans-(−)-kusunokinin and trans-(+)-kusunokinin.
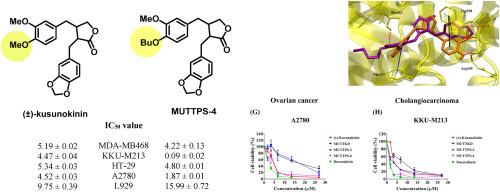
人工合成的 Kusunokinin 类似物对人类癌细胞株的抗癌活性
合成了一系列外消旋草乌甲素衍生物,并研究了它们对乳腺癌(MCF-7、MDA-MB468)、结肠癌(HT-29)、胆管癌(KKU-M213)和卵巢癌(A2780)等癌细胞的细胞毒活性和细胞活力。结果表明,化合物 6aa、6da 和 6de 对乳腺癌(MDA-MB468)、胆管癌(KKU-M213)、结肠癌(HT-29)和卵巢癌(A2780)细胞具有生长抑制作用,IC50 值(μM)分别为 13.分别为 13.77 ± 0.38、7.94 ± 0.45 和 4.22 ± 0.13(MDA-MB468);4.21 ± 0.21、0.97 ± 0.03 和 0.09 ± 0.02(KKU-M213);22.66 ± 0.23 和 15.62 ± 0.06(HT-29);13.11 ± 0.37、11.51 ± 0.43 和 1.87 ± 0.01(A2780)。有趣的是,阳性对照多柔比星对胆管癌 KKU-M213 和卵巢癌 A2780 细胞的细胞毒性低于 6da 和 6de。此外,这三种合成化合物对正常细胞 MMNK-1、Vero 和 L-929 的毒性也低于多柔比星。在与 CSF1R 的结合可能性方面,6de(-11.59 kcal/mol)和反式-(-)-kusunokinin(-11.75 kcal/mol)的对接能量和对接姿势都相似。6de 与 Trp550 通过 π-π 堆积相互作用,与反式-(-)-库苏诺金和反式-(+)-库苏诺金的作用方式相似。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


