Discovery and optimization of tetrahydroacridine derivatives as a novel class of antibiotics against multidrug-resistant Gram-positive pathogens by targeting type I signal peptidase and disrupting bacterial membrane
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Increasing antimicrobial resistance underscores the urgent need for new antibiotics with unique mechanisms. Type I signal peptidase (SPase I) is crucial for bacterial survival and a promising target for antibiotics. Herein we designed and synthesized innovative tetrahydroacridine-9-carboxylic acid derivatives by optimizing the initial hit compound SP11 based on virtual screening. Structure-activity relationship (SAR) studies and bioactivity assessments identified compound C09 as a standout, showing excellent in vitro antimicrobial activity against MRSA and other multidrug-resistant Gram-positive pathogens. C09 targets SPase I with a favorable affinity, disrupts bacterial cell membranes, and eradicates biofilms, reducing resistance risk. In vivo tests in a murine MRSA skin infection model demonstrated significant efficacy. Additionally, C09 has good liver microsome metabolic stability, safe hemolytic activity and mammalian cytotoxicity, as well as a good in vivo safety profile. Overall, our findings highlight the potential of tetrahydroacridine-9-carboxylic acid derivatives as a novel class of antibiotics against multidrug-resistant Gram-positive bacteria.
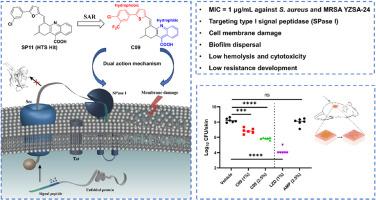
发现并优化四氢吖啶衍生物,将其作为一种新型抗生素,通过靶向 I 型信号肽酶和破坏细菌膜来抗击具有多重耐药性的革兰氏阳性病原体
抗菌药耐药性的不断增加凸显了对具有独特机制的新型抗生素的迫切需求。I 型信号肽酶(SPase I)是细菌生存的关键,也是抗生素的有望靶点。在此,我们在虚拟筛选的基础上,通过优化初始命中化合物 SP11,设计并合成了创新的四氢吖啶-9-羧酸衍生物。通过结构-活性关系(SAR)研究和生物活性评估,我们发现化合物 C09 是一个突出的化合物,它对 MRSA 和其他耐多药革兰氏阳性病原体具有出色的体外抗菌活性。C09 以良好的亲和力靶向 SPase I,破坏细菌细胞膜,清除生物膜,降低耐药性风险。在小鼠 MRSA 皮肤感染模型中进行的体内试验显示了显著的疗效。此外,C09 还具有良好的肝微粒体代谢稳定性、安全的溶血活性和哺乳动物细胞毒性,以及良好的体内安全性。总之,我们的研究结果凸显了四氢吖啶-9-羧酸衍生物作为一类新型抗生素对付耐多药革兰氏阳性菌的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


